Description
• রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন।
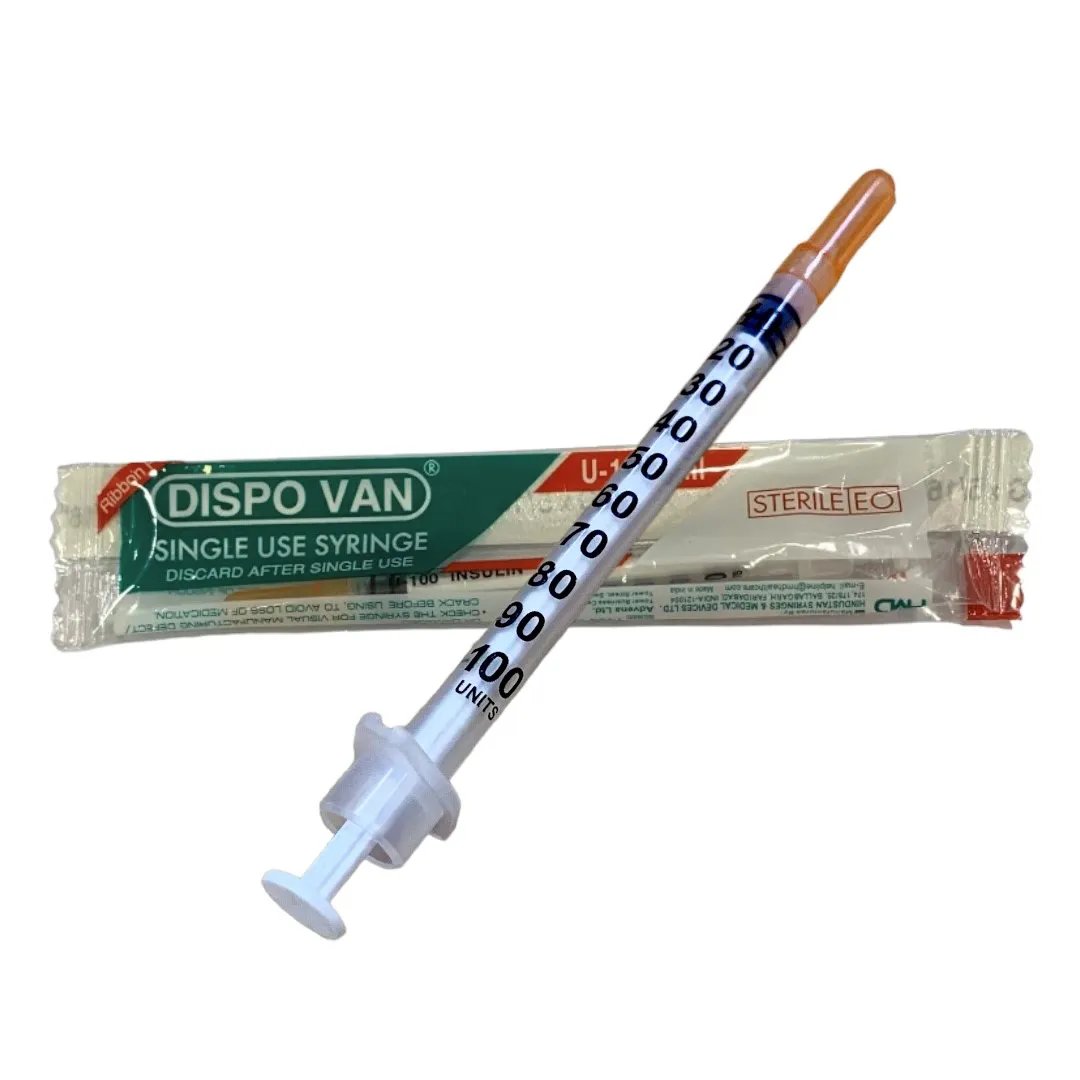
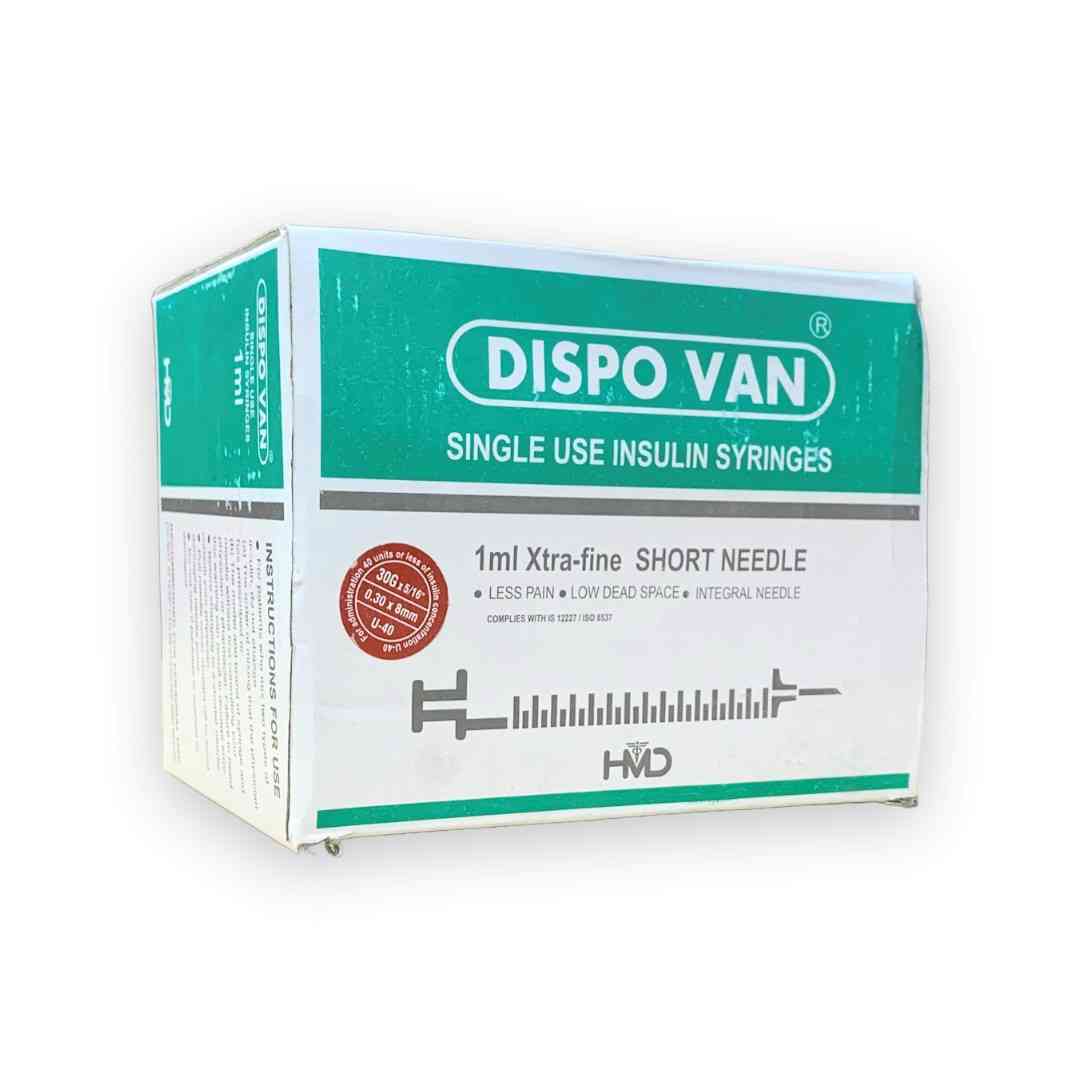
DISPOVAN U40 PCS is used for the safe and accurate administration of U40 insulin to diabetic patients. The syringe is designed for single use, ensuring hygienic and risk-free injection practices. It is an essential tool in the daily management of diabetes, providing ease of use and comfort for individuals needing insulin injections.
Pharmacology:
DISPOVAN U40 PCS is a disposable syringe designed for the administration of U40 insulin, which has a specific concentration of insulin. The syringe features a clear and easily readable scale to accurately measure the insulin dosage, preventing the risk of under or overdosing. It ensures that insulin is delivered safely and precisely into the body for proper blood sugar regulation.
Dosage:
The dosage of insulin administered using the DISPOVAN U40 PCS will depend on the patient's insulin therapy plan as prescribed by their doctor. Typically, the dosage ranges based on individual needs and the type of insulin therapy being followed.
Always follow your healthcare provider's instructions for insulin dosing and use the correct syringe to match the insulin concentration.
Administration:
DISPOVAN U40 PCS should be used for subcutaneous injection of U40 insulin.
-
Before using the syringe, ensure it is sterile and has not been used previously.
-
Draw the prescribed amount of insulin into the syringe, ensuring no air bubbles remain.
-
Inject the insulin as directed by your healthcare provider, usually in the fatty tissue beneath the skin, such as the upper arm or abdomen.
-
Dispose of the syringe immediately after use in a safe, designated disposal container.
Interaction:
DISPOVAN U40 PCS is a medical device, and no direct pharmacological interactions are expected. However, ensure that the insulin used is compatible with your prescribed treatment regimen. Avoid mixing insulin types unless directed by your healthcare provider.
Contraindications:
DISPOVAN U40 PCS is contraindicated for use with other than U40 insulin.
It should not be used if the syringe is damaged, compromised, or if it has been used previously.
This syringe should not be reused to prevent contamination or infection.
Side Effects:
As a medical device, DISPOVAN U40 PCS itself does not have side effects. However, improper injection techniques or the use of expired insulin may lead to complications such as:
-
Hypoglycemia (low blood sugar)
-
Hyperglycemia (high blood sugar)
-
Injection site reactions (redness, swelling, or irritation)
If any unusual side effects occur during insulin administration, consult your healthcare provider immediately.
Pregnancy & Lactation:
DISPOVAN U40 PCS is intended for use in diabetic patients regardless of pregnancy status. However, insulin use during pregnancy should always be done under the supervision of a healthcare provider to adjust the insulin dosage for maternal and fetal health.
For breastfeeding mothers, insulin use does not affect milk production, but blood sugar levels should be monitored carefully.
Therapeutic Class:
Medical Devices (Disposable Syringes for Insulin)
Storage Conditions:
-
Store DISPOVAN U40 PCS in a cool, dry place.
-
Keep syringes in their original packaging until use.
-
Do not store in direct sunlight or near excessive heat.
-
Keep out of the reach of children.
-
Dispose of syringes properly after each use in a puncture-proof container.


 PCS.jpg)

 25G.jpg)
2000ML 2000ML.jpg)

 1 PCS.jpg)



 1.jpg)
 L.jpg)
 1.jpg)
 ALL SIZE.jpg)

 60ML.jpg)
 ..jpg)

 1.jpg)


 S.jpg)

 20G.jpg)

 PCS.jpg)
 10CC.jpg)

 ..jpg)
 ..jpg)
 14 CH.jpg)


 22G.jpg)


 1 PAIR 6.jpg)

 PCS.jpg)
 6INC.jpg)

 3CC.jpg)

 PCS.jpg)

 L.jpg)