Description
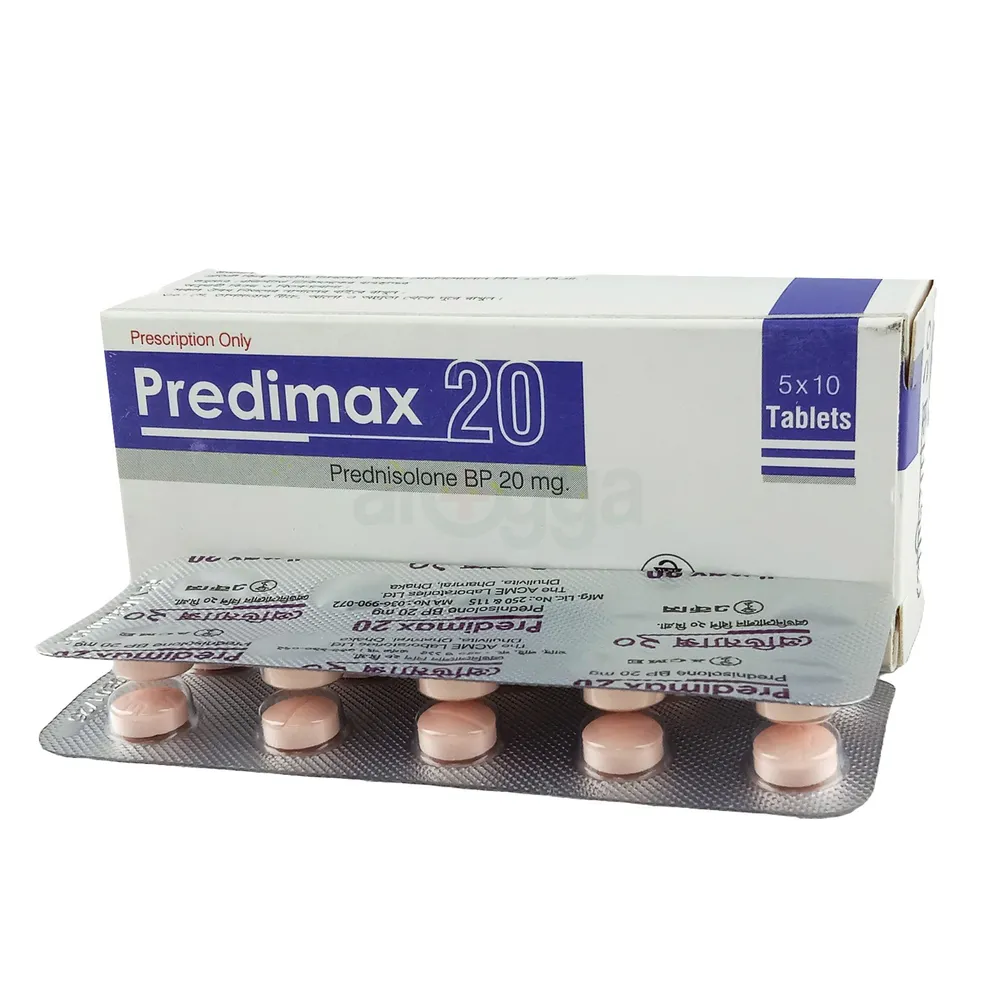
Azin 250 mg is a macrolide antibiotic manufactured by ACME Laboratories Ltd., containing Azithromycin Dihydrate 250 mg per capsule. Azithromycin is known for its broad-spectrum activity against various bacterial pathogens. It works by binding to the 50S ribosomal subunit of susceptible bacteria, thereby inhibiting protein synthesis and halting bacterial growth. Azithromycin is acid-stable and can be taken orally without the need for protection from gastric acids. Its absorption is greater on an empty stomach, and peak plasma concentrations are typically reached within 2.1 to 3.2 hours after oral administration. Due to its high concentration in phagocytes, azithromycin is actively transported to the site of infection, where it is released during active phagocytosis. The concentration of azithromycin in tissues can be over 50 times higher than in plasma, owing to ion trapping and high lipid solubility. This extensive tissue penetration allows for a large single dose to maintain bacteriostatic levels in the infected tissue for several days. Following a single 500 mg dose, plasma concentrations of azithromycin decline in a polyphasic pattern, with a terminal elimination half-life of approximately 68 hours. The prolonged half-life is thought to be due to extensive uptake and subsequent release of the drug from tissues. Biliary excretion of azithromycin, predominantly unchanged, is a major route of elimination. Over the course of a week, approximately 6% of the administered dose appears as unchanged drug in urine.


.png)
























.png)
.webp)


.jpeg)



.webp)









.png)












.webp)

















.jpeg)








.jpeg)