Description
রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
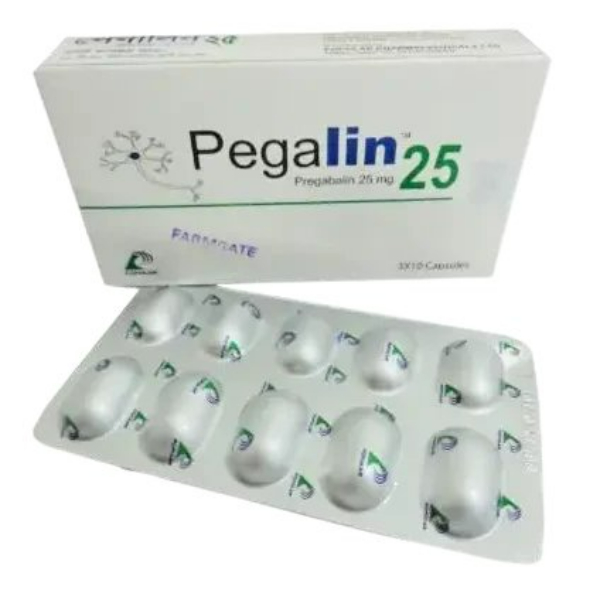
Indications: PEGALIN (Pregabalin) is indicated for:
- Neuropathic pain associated with diabetic peripheral neuropathy (DPN) in adults.
- Postherpetic neuralgia (PHN), the pain that follows shingles.
- Adjunctive therapy for the treatment of partial-onset seizures in adults.
- Management of fibromyalgia.
- Neuropathic pain associated with spinal cord injury.
- Generalized anxiety disorder (though less common in some regions for this indication).
Pharmacology:
Pregabalin is a structural derivative of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA).
Dosage: The dosage of PEGALIN 25 MG should be individualized based on patient response and tolerability, and always as prescribed by a registered physician.
- For Neuropathic pain (e.g., Diabetic peripheral neuropathy, Postherpetic neuralgia, Spinal cord injury):
- Initial dose is typically 75 mg two times a day (150 mg/day) or 50 mg three times a day (150 mg/day).
- The dose may be increased based on efficacy and tolerability, usually within one week, up to a maximum of 300 mg/day initially.
- Further increases up to a maximum of 600 mg/day (e.g., 300 mg twice a day or 200 mg three times a day) may be considered if needed, under medical supervision.
- For Fibromyalgia:
- Recommended dose range is 300 to 450 mg/day.
- Dosing should begin at 75 mg two times a day (150 mg/day) and may be increased to 150 mg two times a day (300 mg/day) within 1 week.
If needed, the dose can be increased to 225 mg two times a day (450 mg/day).
- For Partial-onset seizures (adjunctive therapy):
- Initial dose is generally no more than 150 mg/day (e.g., 75 mg two times a day or 50 mg three times a day).
- The dose may be increased up to a maximum of 600 mg/day based on individual response and tolerability.
Administration:
PEGALIN capsules can be taken orally with or without food.
Interaction:
- CNS Depressants: Concomitant use of Pregabalin with other CNS depressants (e.g., alcohol, opioids, benzodiazepines, hypnotics, anxiolytics) may result in additive CNS depression, leading to increased drowsiness, sedation, and respiratory depression.
- Thiazolidinedione Antidiabetic Agents: Co-administration with thiazolidinedione antidiabetic agents may increase the risk of peripheral edema and weight gain.
- ACE Inhibitors: May increase the risk of angioedema when used with ACE inhibitors.
- No significant pharmacokinetic interactions with other antiepileptic drugs or oral contraceptives have been reported.
Contraindications:
PEGALIN is contraindicated in patients with known hypersensitivity to Pregabalin or any of its excipients.
Side Effects: The most common side effects include:
- Dizziness
- Somnolence (drowsiness/sleepiness)
- Dry mouth
- Edema (swelling, particularly of hands and feet)
- Blurred vision, double vision (diplopia)
- Weight gain
- Difficulty concentrating or paying attention
- Fatigue
- Headache
- Increased appetite
- Ataxia (lack of coordination)
- Tremor
- Nausea, vomiting, constipation, flatulence
- Euphoria (feeling "high")
- Irritability
- Suicidal thoughts or behavior (rare but serious; monitor for changes in mood or behavior)
- Angioedema (swelling of face, lips, tongue, throat - may require emergency medical attention)
- Respiratory depression (especially with other CNS depressants or underlying respiratory impairment)
- Muscle pain, tenderness, or weakness (especially if accompanied by fever)
If any serious or persistent side effects occur, or if you experience symptoms like hives, blisters, difficulty breathing, or swelling of face/throat, seek immediate medical attention.
Pregnancy & Lactation:
Pregnancy: Pregabalin is classified as Pregnancy Category C.
Precautions & Warnings:
- Withdrawal Symptoms: Do not discontinue PEGALIN abruptly. Sudden discontinuation may lead to withdrawal symptoms such as insomnia, nausea, headache, diarrhea, anxiety, agitation, nervousness, depression, and seizures.
Dosage should be tapered gradually over at least 1 week. - Dizziness and Somnolence: Pregabalin may cause dizziness and somnolence.
Patients should be advised not to drive, operate complex machinery, or engage in other hazardous activities until they are certain that Pregabalin does not adversely affect their ability to perform such activities. - Renal Impairment: Dosage adjustments are necessary for patients with impaired renal function, as Pregabalin is primarily eliminated by the kidneys.
- Angioedema: Cases of angioedema (swelling of the face, mouth, lips, gums, tongue, neck, and throat) have occurred. If this happens, discontinue the drug immediately and seek emergency medical attention.
- Hypersensitivity: Hypersensitivity reactions (e.g., hives, dyspnea, wheezing) have been reported. Discontinue immediately if such reactions occur.
- Suicidal Thoughts: Antiepileptic drugs, including Pregabalin, may increase the risk of suicidal thoughts or behavior. Patients should be monitored for new or worsening depression, suicidal thoughts or behavior, or any unusual changes in mood or behavior.
- Weight Gain and Edema: Weight gain and peripheral edema have been observed, especially in patients with diabetes or heart problems.
Caution should be exercised in patients with cardiovascular disease. - Vision Changes: Blurred vision or other visual disturbances may occur.
If vision changes develop, consult a doctor. - Drug Abuse Potential: Pregabalin has a potential for abuse and dependence.
Use with caution in patients with a history of substance abuse. - Children and Adolescents: Safety and efficacy in pediatric patients (below a certain age, usually 17 or 18, depending on the indication) have not been fully established for all indications.
Therapeutic Class: Adjunct anti-epileptic drugs, Primary anti-epileptic drugs, Neuropathic pain agents, Fibromyalgia agents.
Storage Conditions:
Store below 30°C in a cool, dry place, away from direct sunlight and moisture.






























































.jpg)