Description
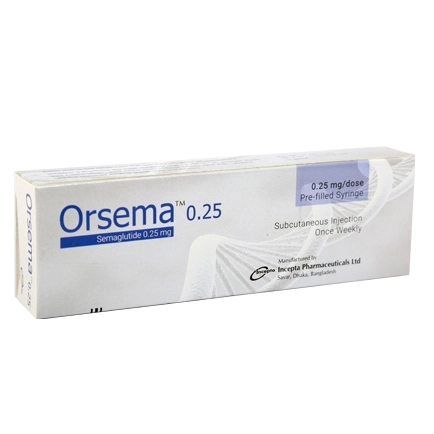
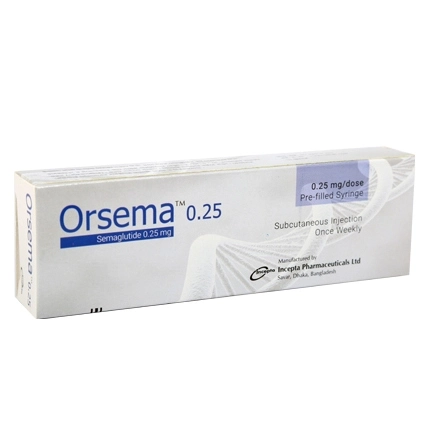
Orsema 0.25 mg is a semaglutide-based injectable medication developed by Incepta Pharmaceuticals Ltd., primarily used for managing type 2 diabetes mellitus. As a GLP-1 receptor agonist, semaglutide mimics the action of the natural hormone GLP-1, enhancing insulin secretion and suppressing glucagon release in a glucose-dependent manner, thereby aiding in blood sugar regulation. Additionally, it slows gastric emptying and reduces appetite, contributing to weight loss, which is beneficial for individuals with obesity or weight-related comorbidities. Administered once weekly via subcutaneous injection in the abdomen, thigh, or upper arm, Orsema 0.25 mg serves as the initial dose in a titration schedule aimed at minimizing gastrointestinal side effects. Common adverse effects include nausea, vomiting, diarrhea, and abdominal discomfort, while serious risks encompass pancreatitis, thyroid C-cell tumors, and diabetic retinopathy complications. Orsema is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. Proper storage involves refrigeration between 2°C and 8°C, avoiding freezing. This medication is available in Bangladesh and should be used under medical supervision as part of a comprehensive diabetes management plan














.jpg)

.jpg)




.jpg)

























.jpg)










.jpeg)
.webp)

.jpg)


.png)


 400MG.jpg)




















.jpg)
 100 IU 3 ML.webp)




























.jpg)





.jpeg)





.jpeg)












.jpeg)










.jpg)




















.webp)











.webp)