Description
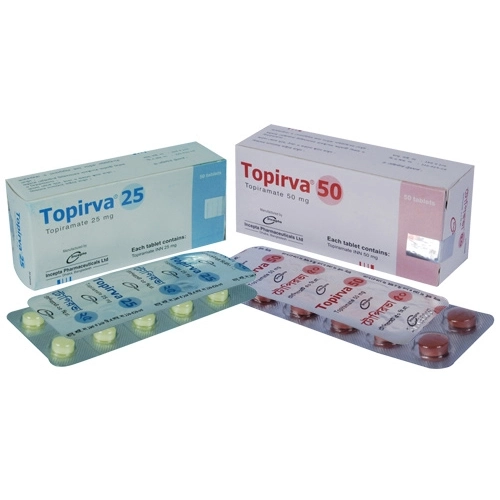
Topirva XR 25 mg Capsule is an extended-release formulation of Topiramate, developed by Incepta Pharmaceuticals Ltd., designed for the management of epilepsy and migraine prophylaxis. Topiramate is a sulfamate-substituted monosaccharide that modulates neuronal excitability by enhancing GABA-A receptor activity and inhibiting excitatory neurotransmission through actions on kainate and AMPA receptors. It also weakly inhibits carbonic anhydrase, contributing to its therapeutic effects. In epilepsy, Topirva XR is indicated as monotherapy for adults and children aged 6 years and above with newly diagnosed epilepsy, including generalized tonic-clonic seizures and partial seizures with or without secondary generalization. It is also used as adjunctive therapy for adults and children over 2 years of age who have not achieved adequate control with conventional first-line antiepileptic drugs. In migraine prophylaxis, Topirva XR is recommended for adults experiencing frequent migraine attacks that significantly interfere with daily activities. The extended-release formulation allows for once-daily dosing, improving patient compliance. The recommended initial dose is 25 mg nightly for 1 week, with gradual titration to a target dose of 100 mg/day, administered in two divided doses. Common side effects may include dizziness, fatigue, weight loss, and cognitive disturbances. It is important to use Topirva XR under the supervision of a healthcare provider, as dosage adjustments may be necessary based on individual response and tolerability



 400MG.jpg)


.jpeg)
















.webp)













.jpg)




.jpg)















.webp)














.jpeg)









.jpg)



.jpg)






.jpg)































.jpg)










 100 IU 3 ML.webp)








.jpg)








.jpeg)
.jpg)









.webp)
.jpeg)





.png)