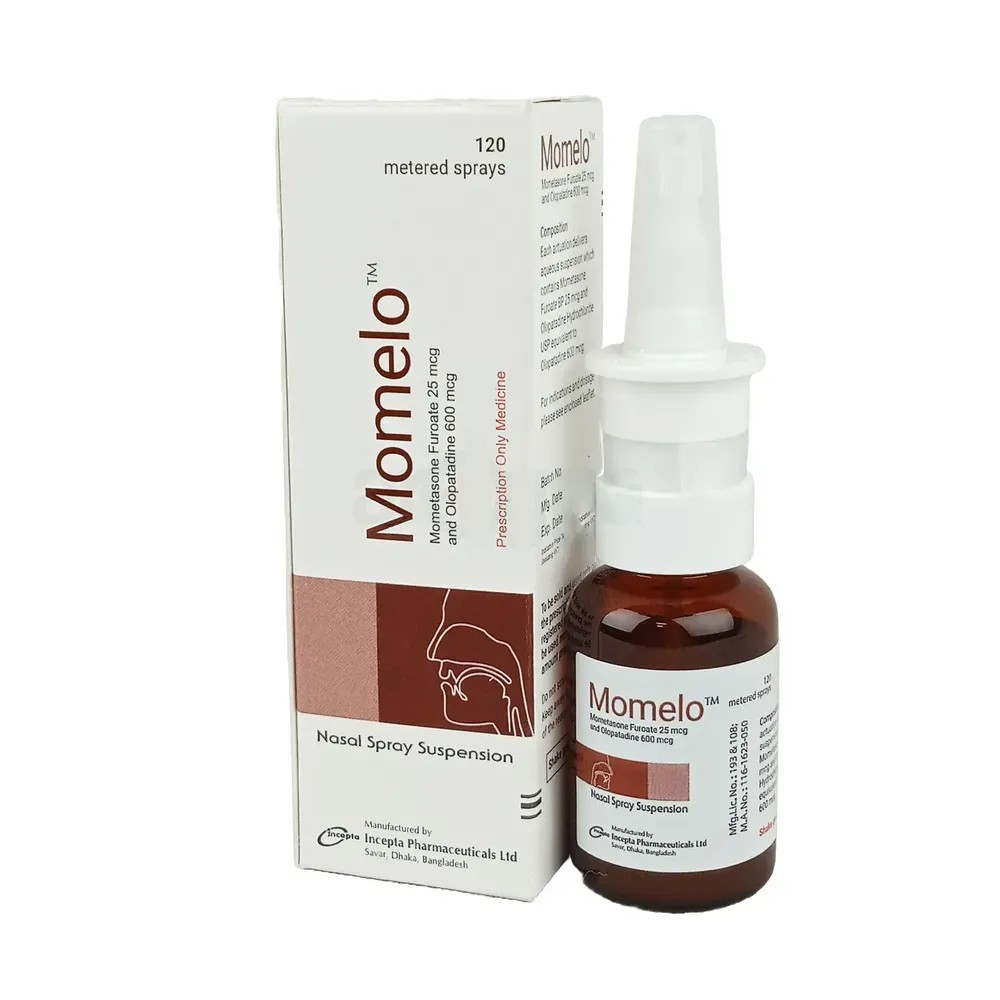
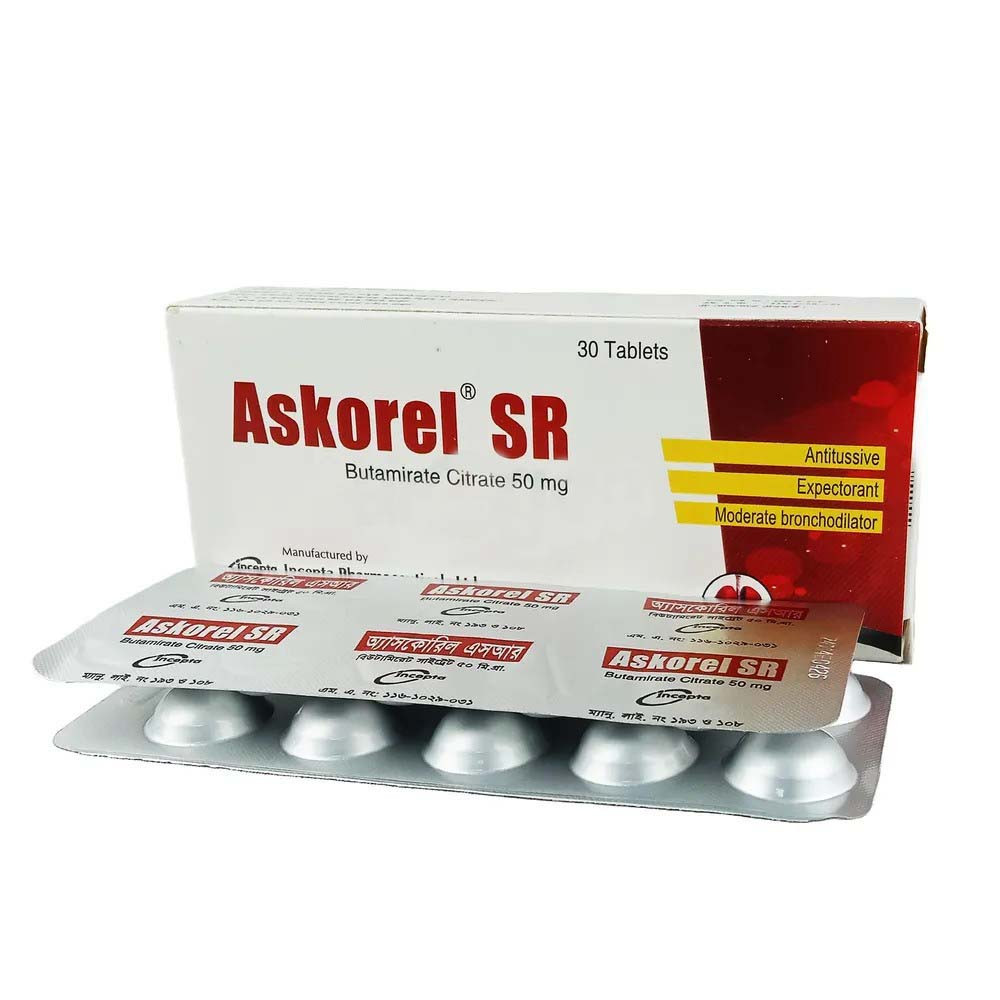
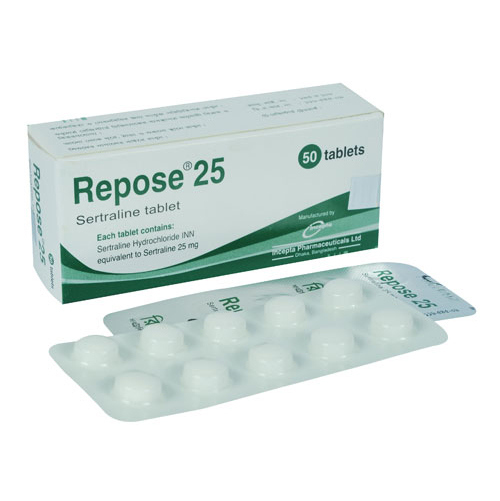
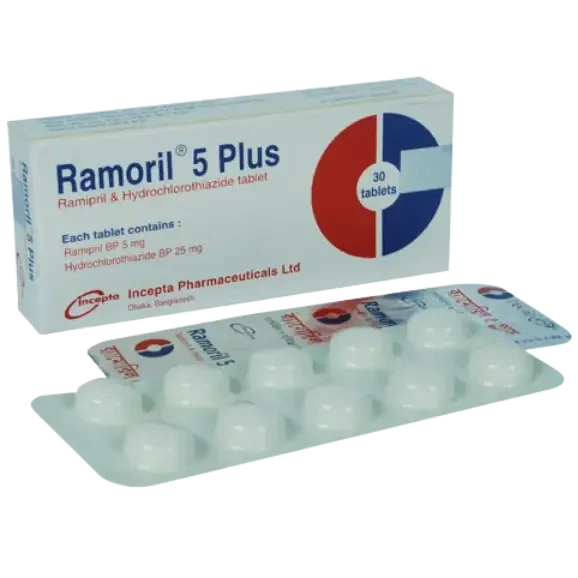
Description
Timopress 0.5% Ophthalmic Solution is a sterile eye drop manufactured by Incepta Pharmaceuticals Ltd., containing Timolol Maleate 0.5% as the active ingredient. Timolol is a non-selective beta-adrenergic receptor blocker that reduces elevated intraocular pressure by decreasing the production of aqueous humor in the eye. It is primarily indicated for the treatment of ocular hypertension and open-angle glaucoma. The usual dosage involves instilling one drop of the 0.25% solution twice daily into the affected eye(s), which may be increased to one drop of the 0.5% solution twice daily if there is inadequate response; the dosage may be decreased to one drop once daily if controlled. Common side effects include burning and stinging sensation of the eyes, bradycardia, hypotension, arrhythmia, headache, dizziness, fatigue, abdominal discomfort, nausea, constipation, and hypoglycemia. Timopress should not be used in patients with bronchial asthma, a history of bronchial asthma, severe chronic obstructive pulmonary disease, sinus bradycardia, second or third-degree atrioventricular block, overt cardiac failure, cardiogenic shock, or hypersensitivity to any component of this product. It is important to consult a healthcare provider before using Timopress, especially for individuals with heart or respiratory conditions, and during pregnancy or breastfeeding. The product should be stored between 15–30°C, protected from light, and kept out of reach of children.










.jpg)

































.jpg)







.webp)


.jpg)

.jpeg)


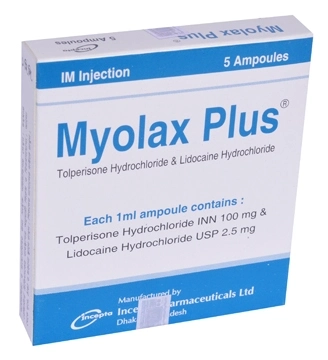
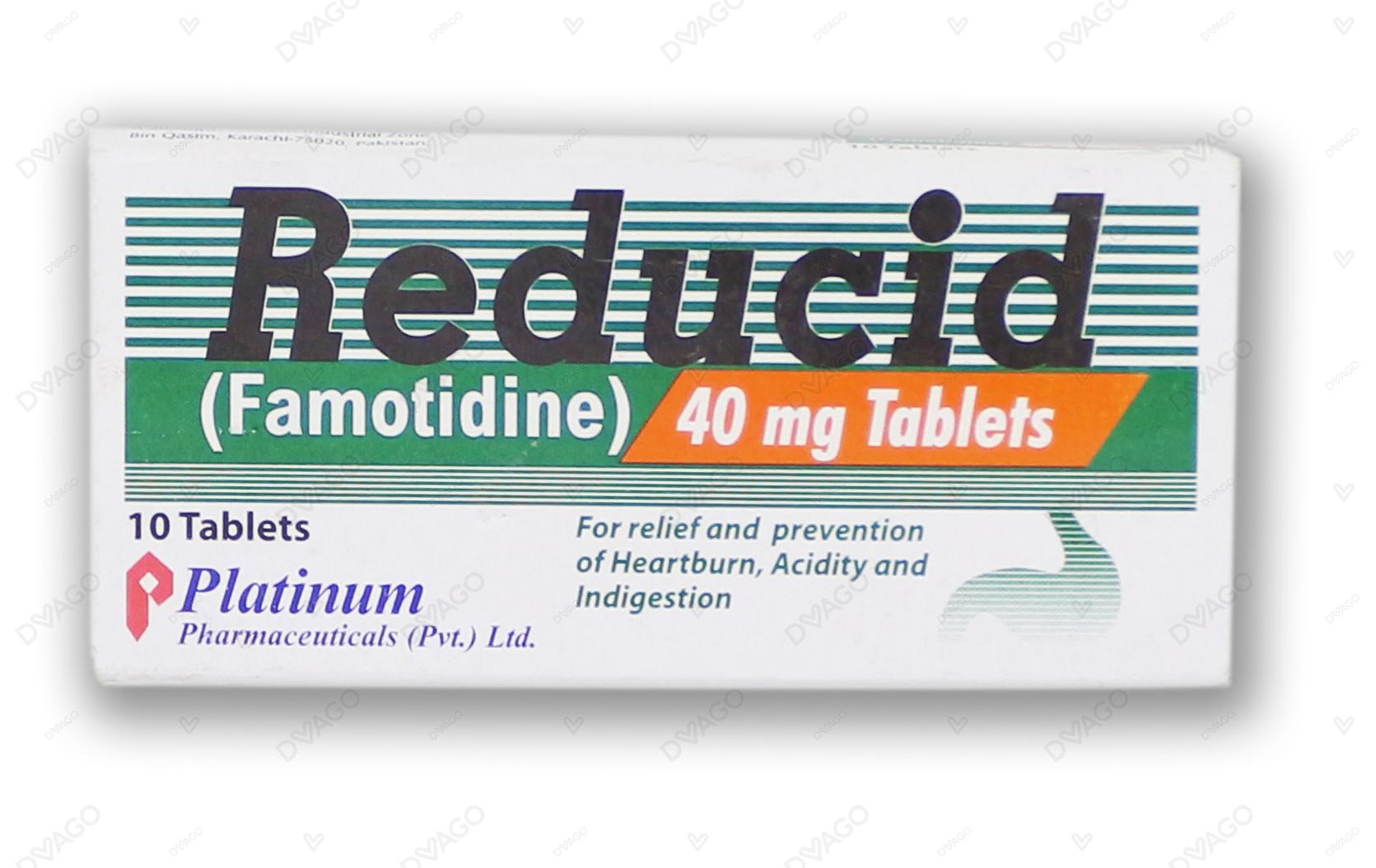
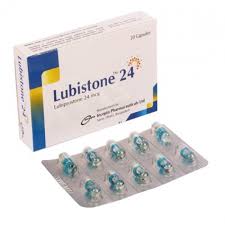

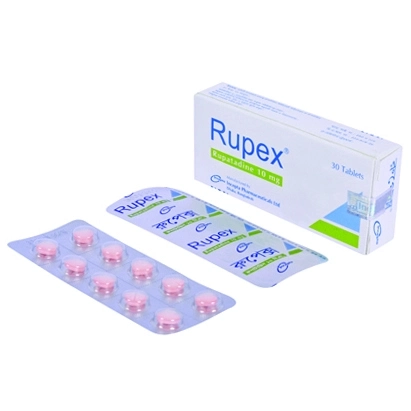






.jpg)






















.jpeg)

























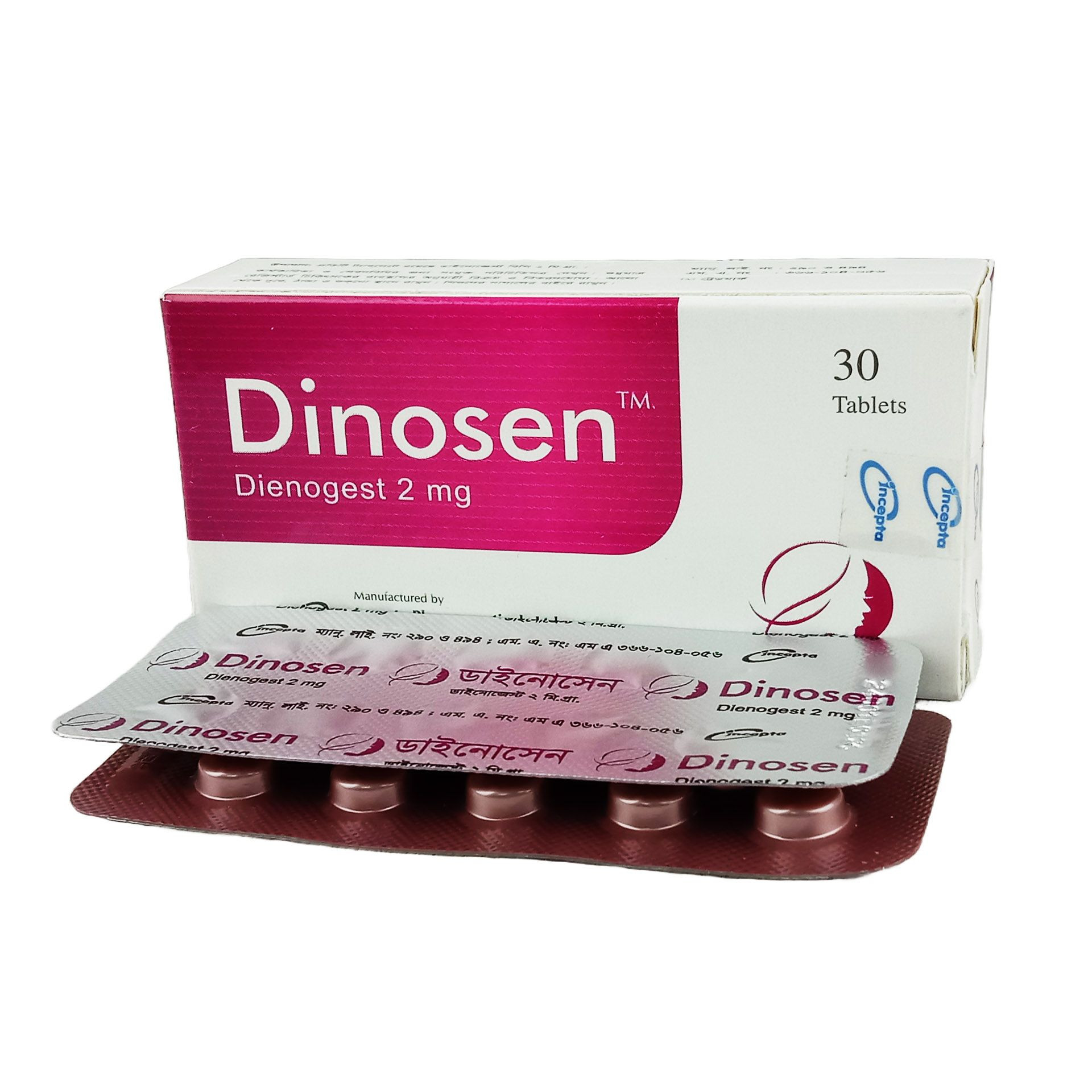
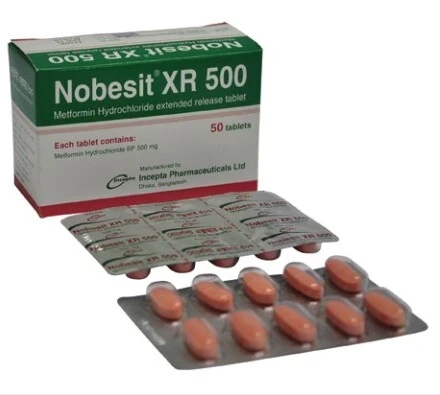
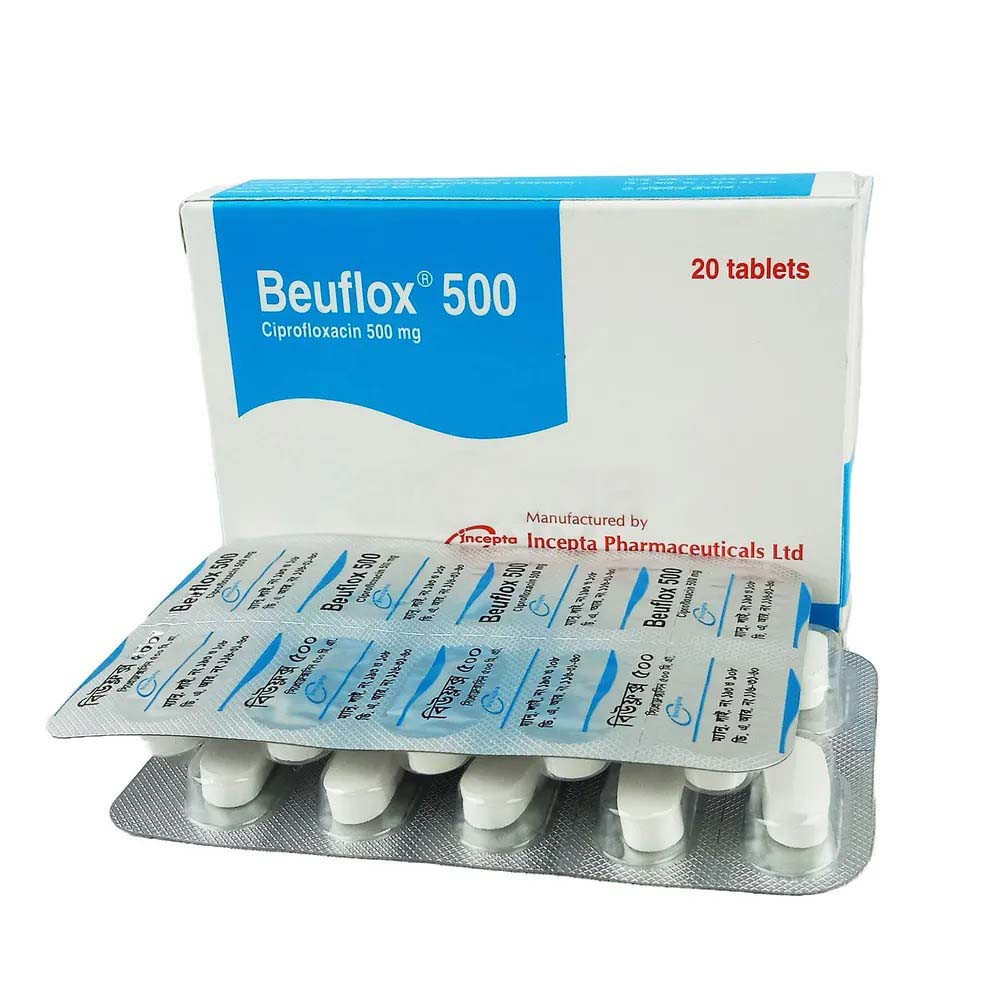


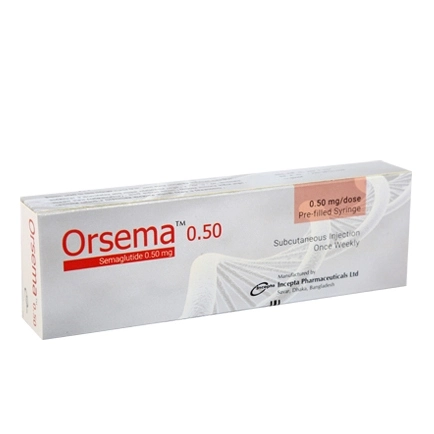











.jpg)


.jpg)
.png)







.webp)








.jpg)






.jpeg)



.jpeg)
.webp)



 400MG.jpg)






















 100 IU 3 ML.webp)

.jpg)