Description
Siesta is a 3 mg tablet manufactured by Incepta Pharmaceuticals Ltd., containing the active ingredient Bromazepam, a pyridylbenzodiazepine with anxiolytic properties. At lower doses, it effectively reduces anxiety, tension, and nervousness, while at higher doses, it exhibits sedative and muscle-relaxant effects. Siesta is indicated for various conditions, including emotional disturbances such as acute anxiety and tension, agitation, insomnia, and anxiety-related depressive reactions. It also addresses functional disturbances in the cardiovascular and respiratory systems, such as pseudoangina pectoris, tachycardia, and dyspnea, as well as gastrointestinal issues like irritable bowel syndrome and epigastric pain. Additionally, it is used for disturbances in the urinary tract, including frequency and dysmenorrhea, and psychosomatic disorders like psychogenic headache and gastric ulcers. The standard dosage for outpatient therapy is 1.5–3 mg up to three times daily, with higher doses administered in severe cases under medical supervision. Treatment duration typically does not exceed 8–12 weeks, and in certain cases, it may be extended with careful monitoring. Elderly patients and those with impaired hepatic function require lower doses. Siesta should be used with caution in children and is generally not recommended unless deemed appropriate by a physician. Common side effects include fatigue, drowsiness, muscle weakness, confusion, and headache. It is contraindicated in patients with known hypersensitivity to bromazepam, severe respiratory insufficiency, severe hepatic insufficiency, or sleep apnea syndrome. Caution is advised when combining Siesta with other centrally active drugs, as its sedative effects may be enhanced. Concomitant intake of Siesta with alcohol should be avoided due to the risk of intensified sedative effects. As with other benzodiazepines, prolonged use may lead to physical and psychological dependence, and abrupt discontinuation should be avoided to prevent withdrawal symptoms. Siesta is not recommended for the primary treatment of sleeplessness caused by psychotic illness. Patients should exercise caution when engaging in activities requiring mental alertness, such as driving or operating machinery. Siesta should be stored in a dry place away from light and heat, out of the reach of children.





.png)









.jpeg)


.jpg)












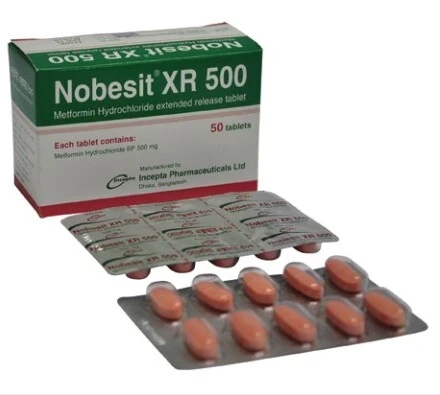

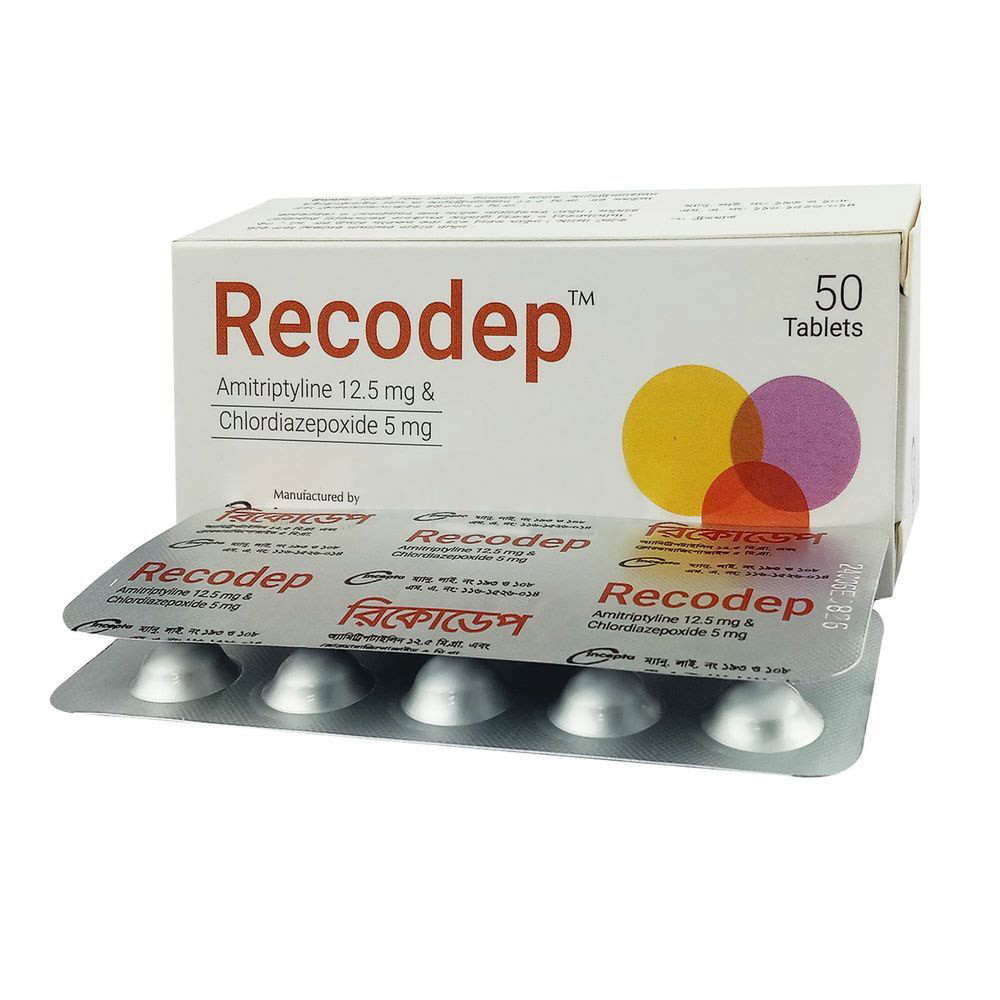
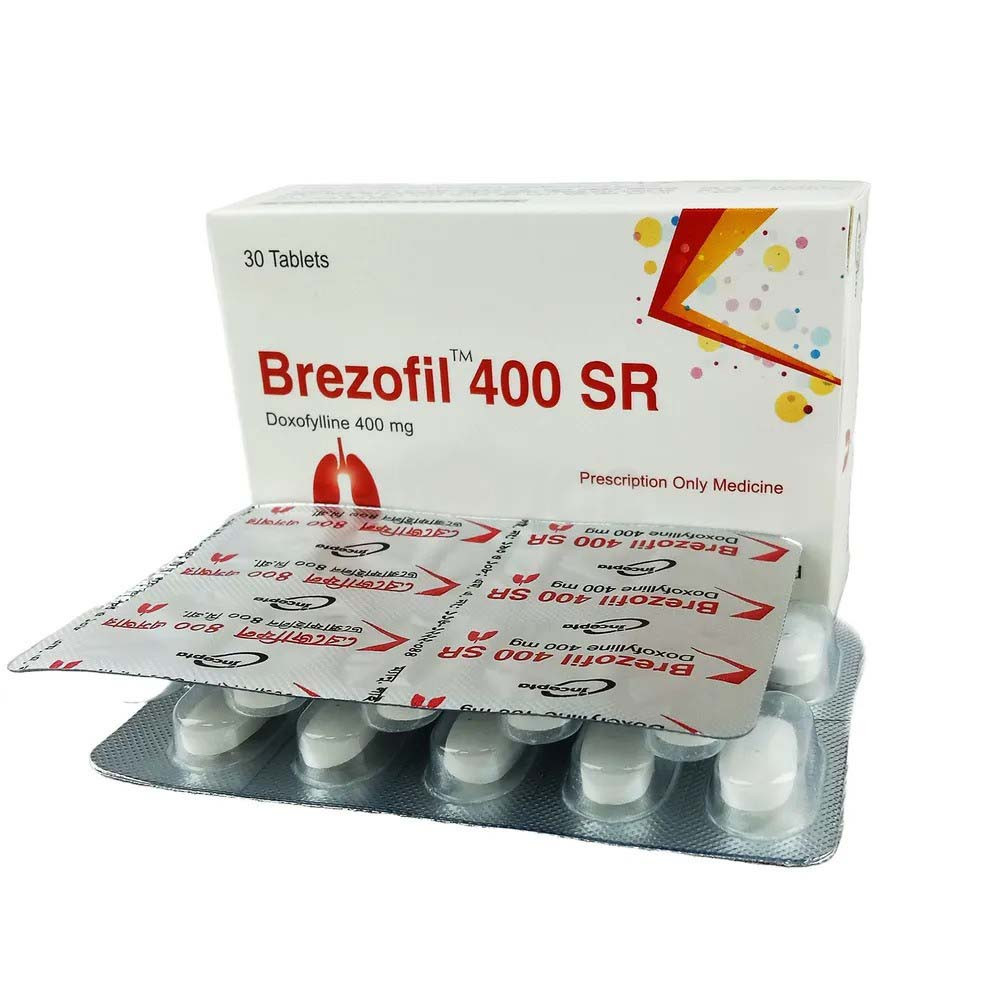
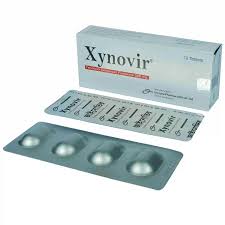




.jpg)


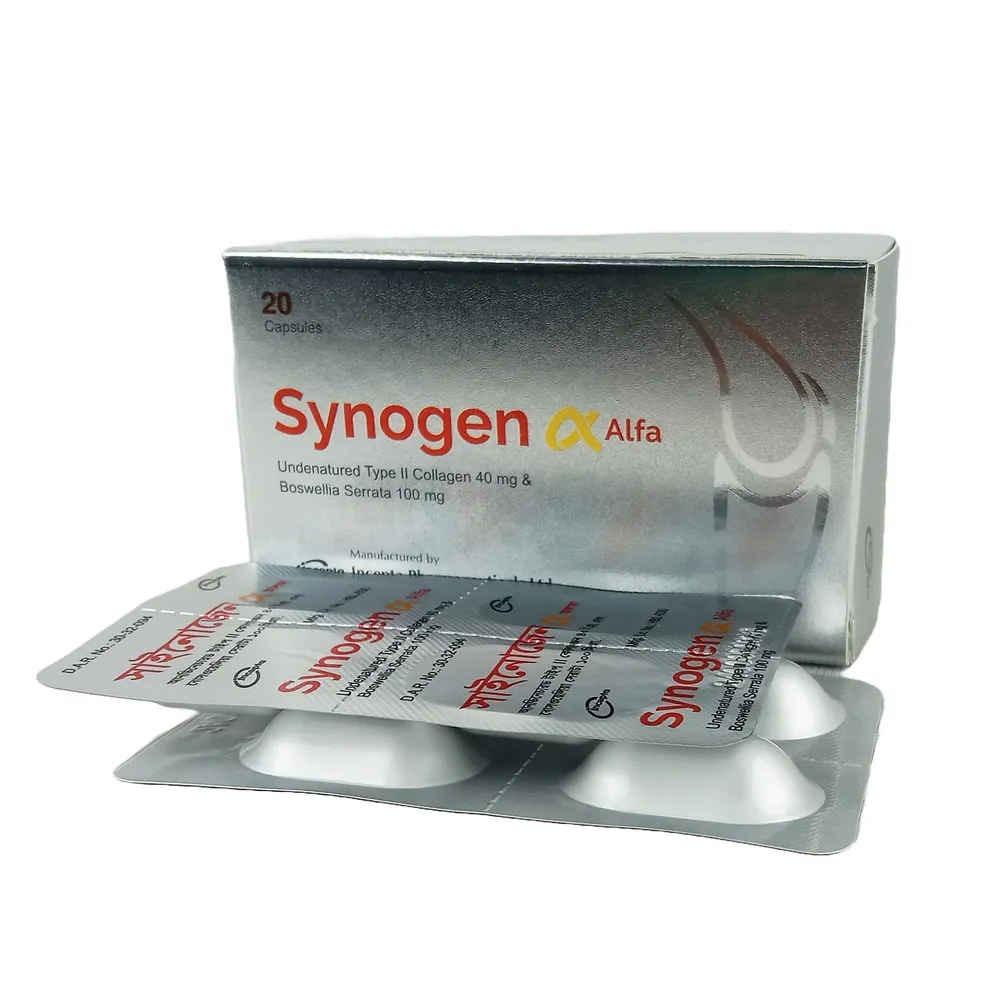

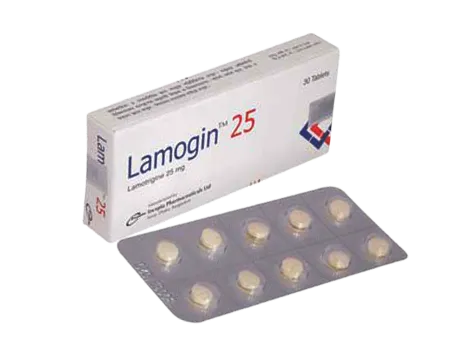
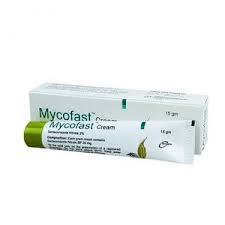
.webp)




 100 IU 3 ML.webp)













.webp)


.jpeg)




.jpg)









.jpg)

 400MG.jpg)





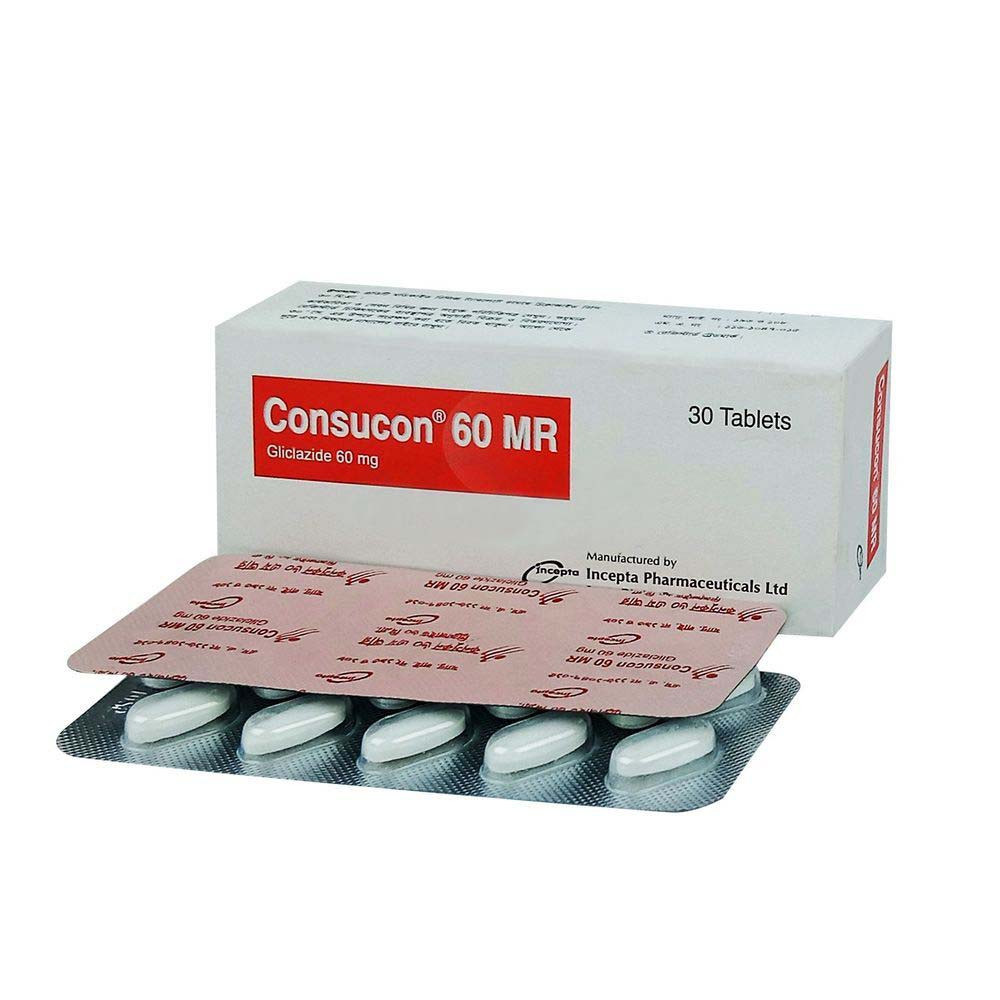

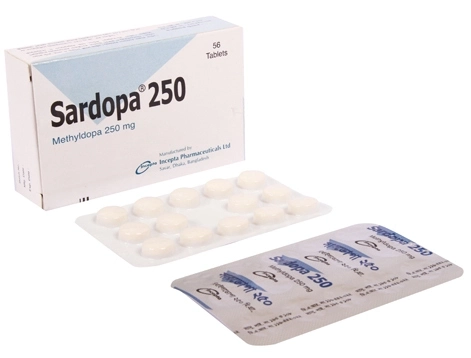
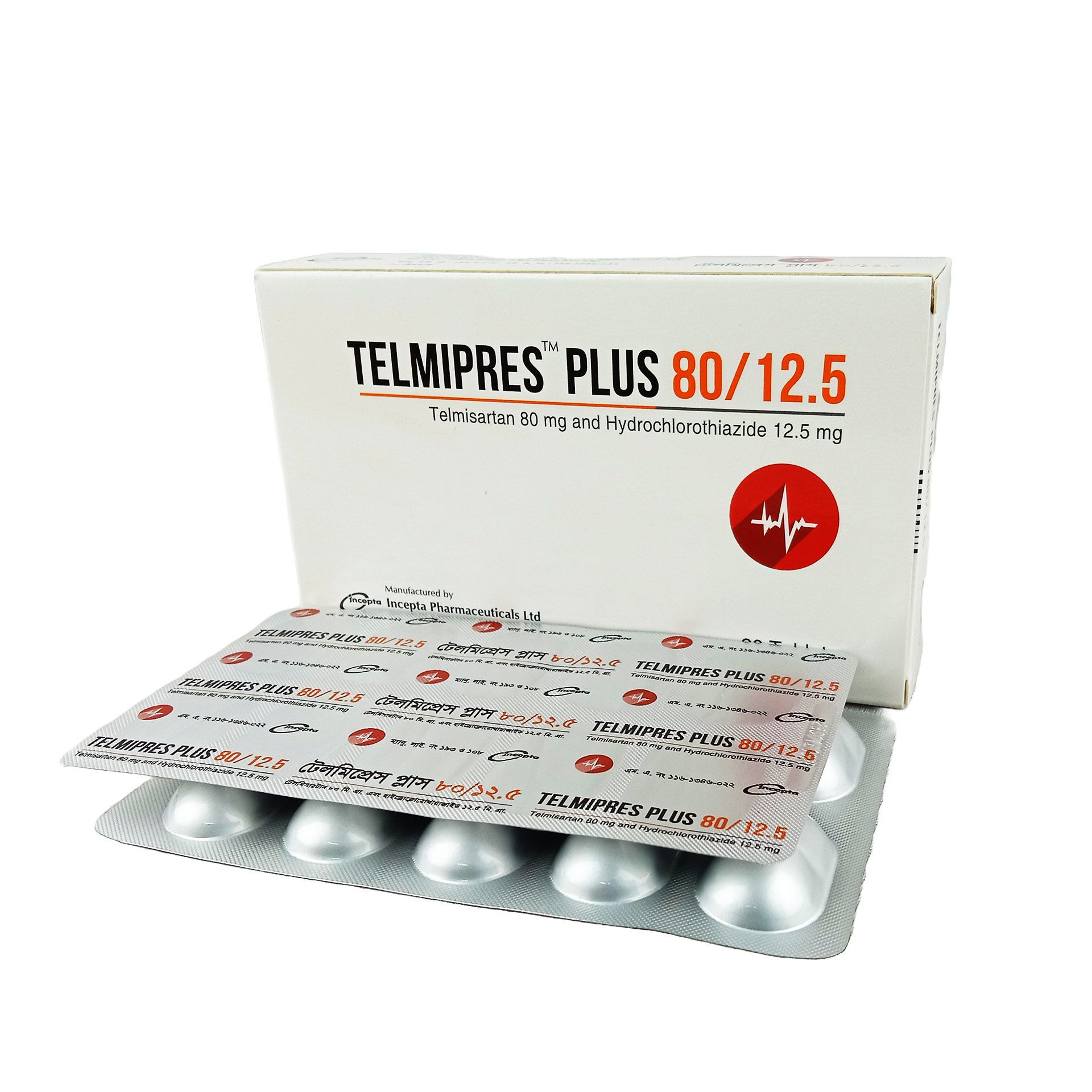
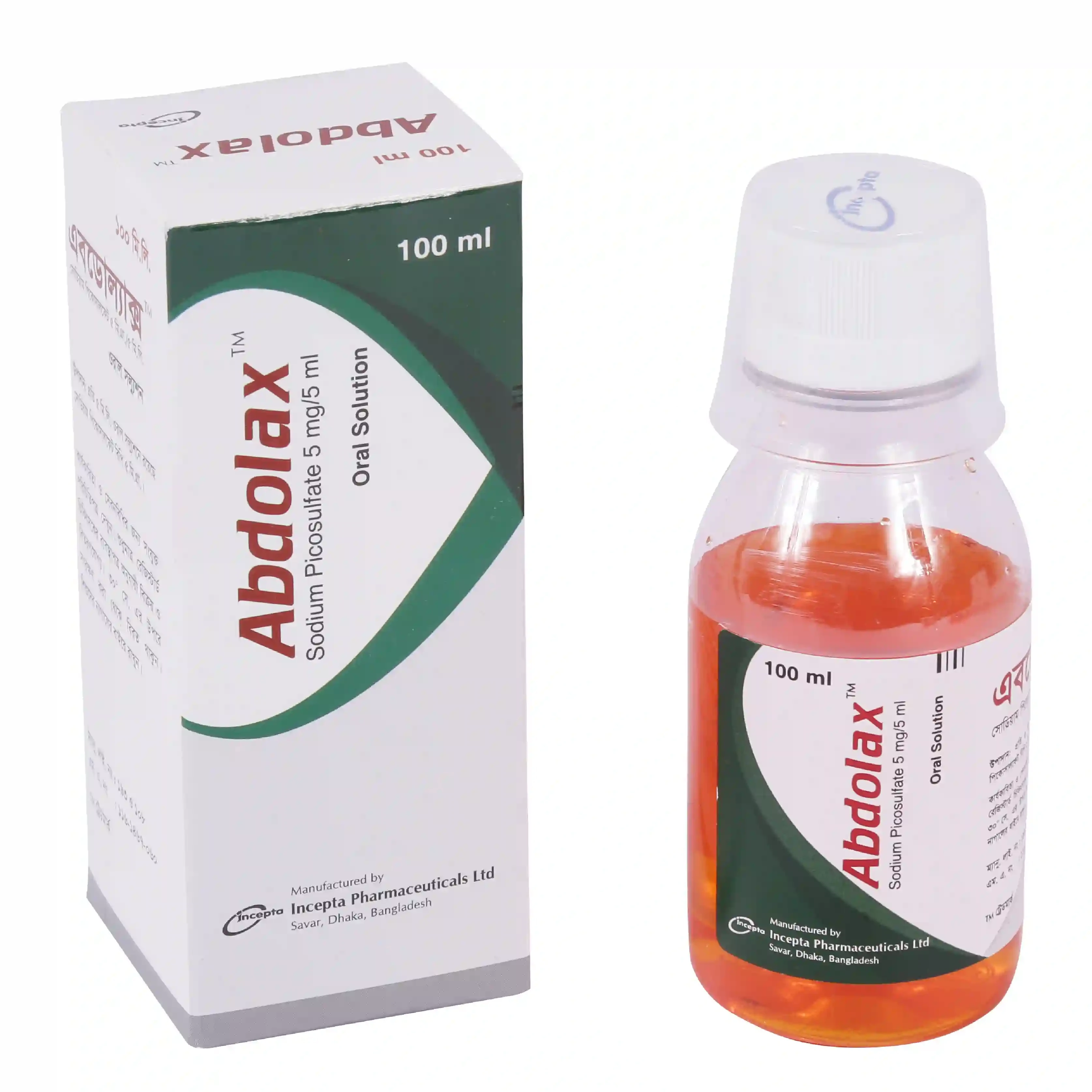










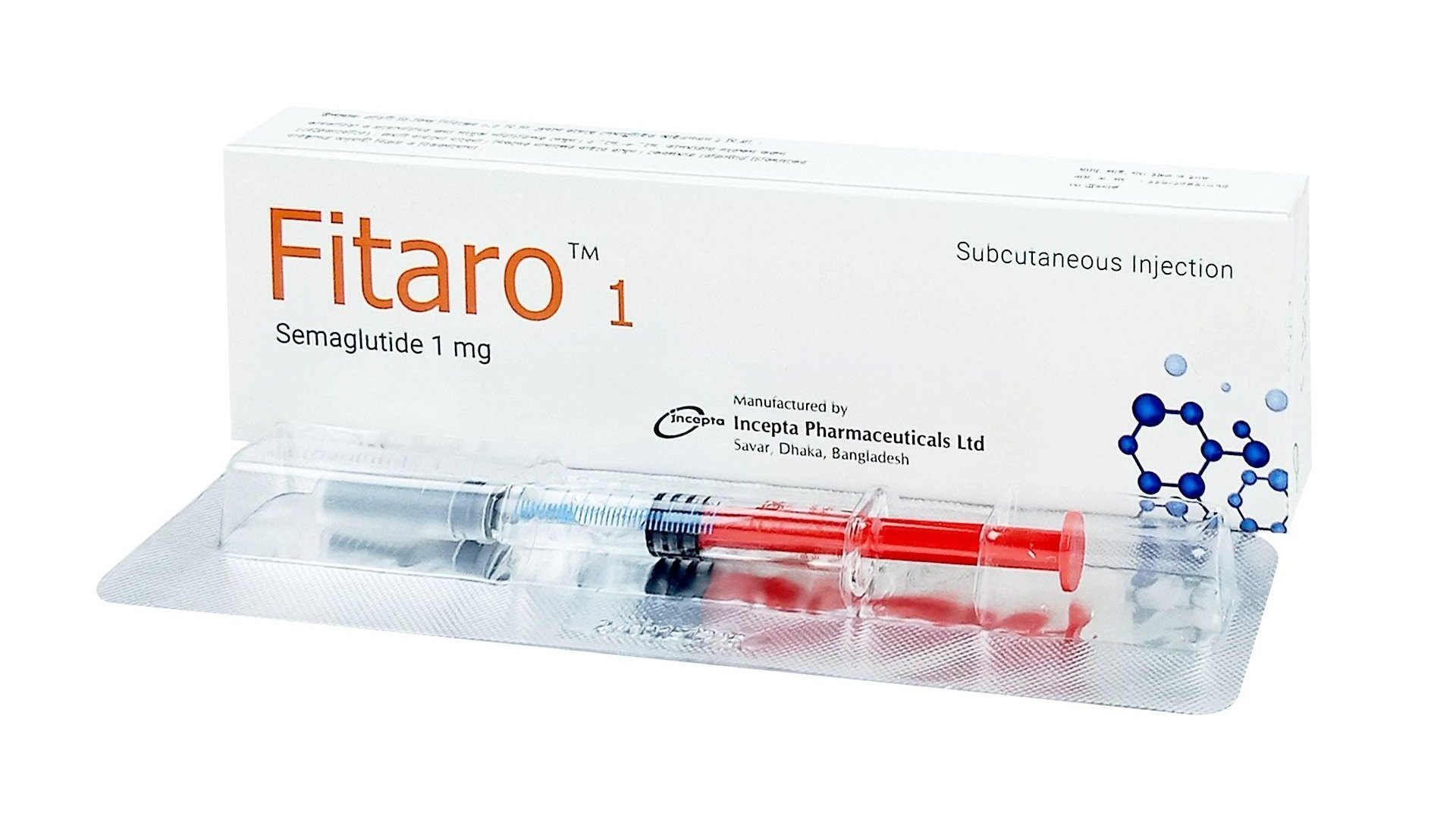





.jpg)























.jpg)
.jpeg)



.webp)







.jpg)

















.jpeg)
















.jpg)