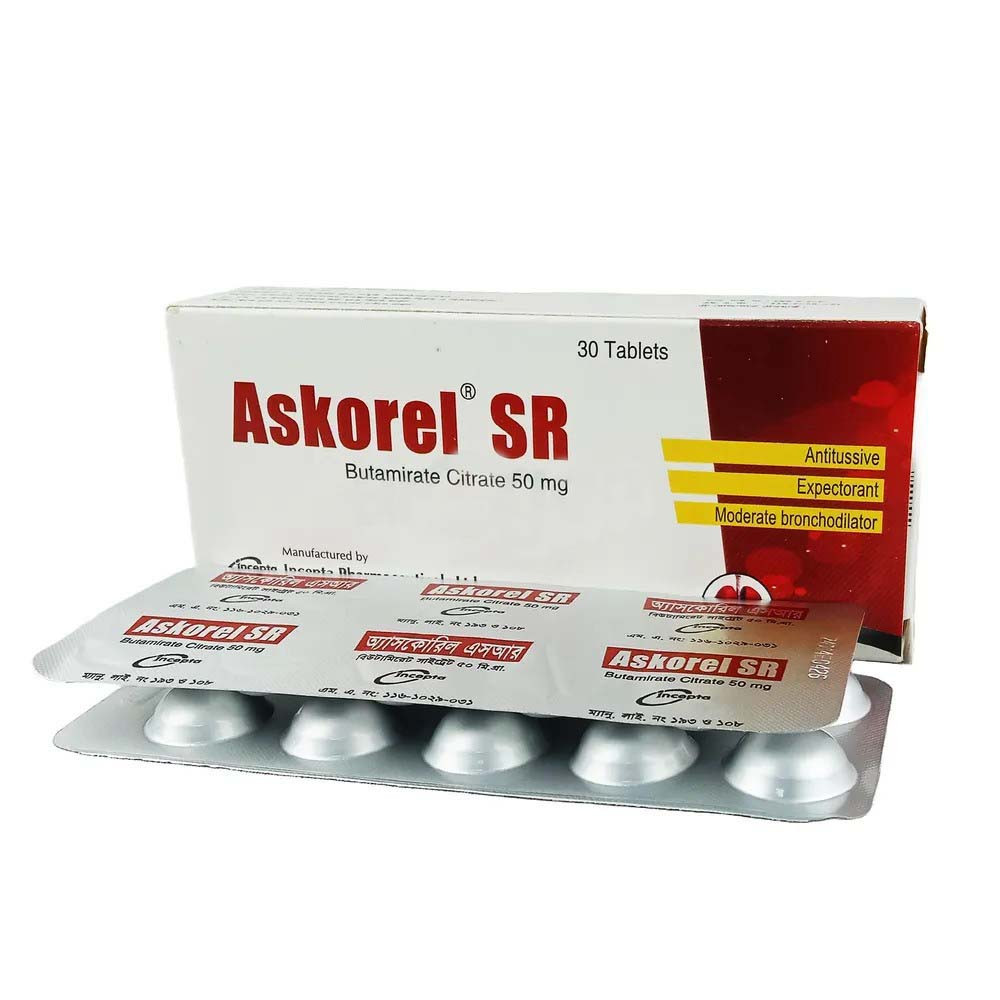
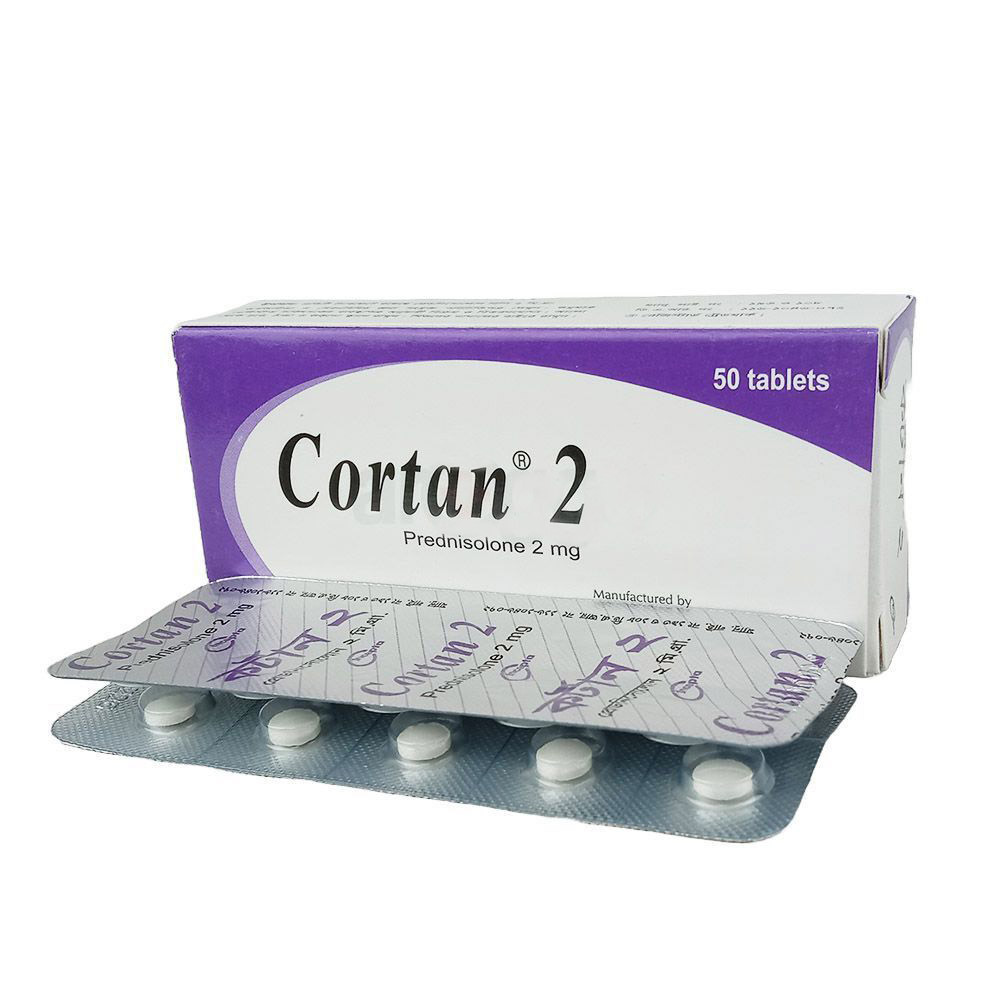
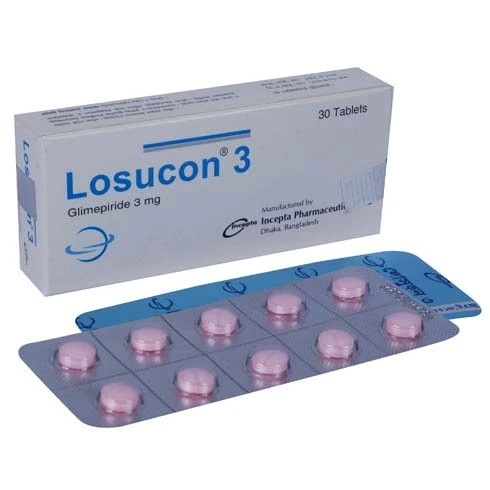
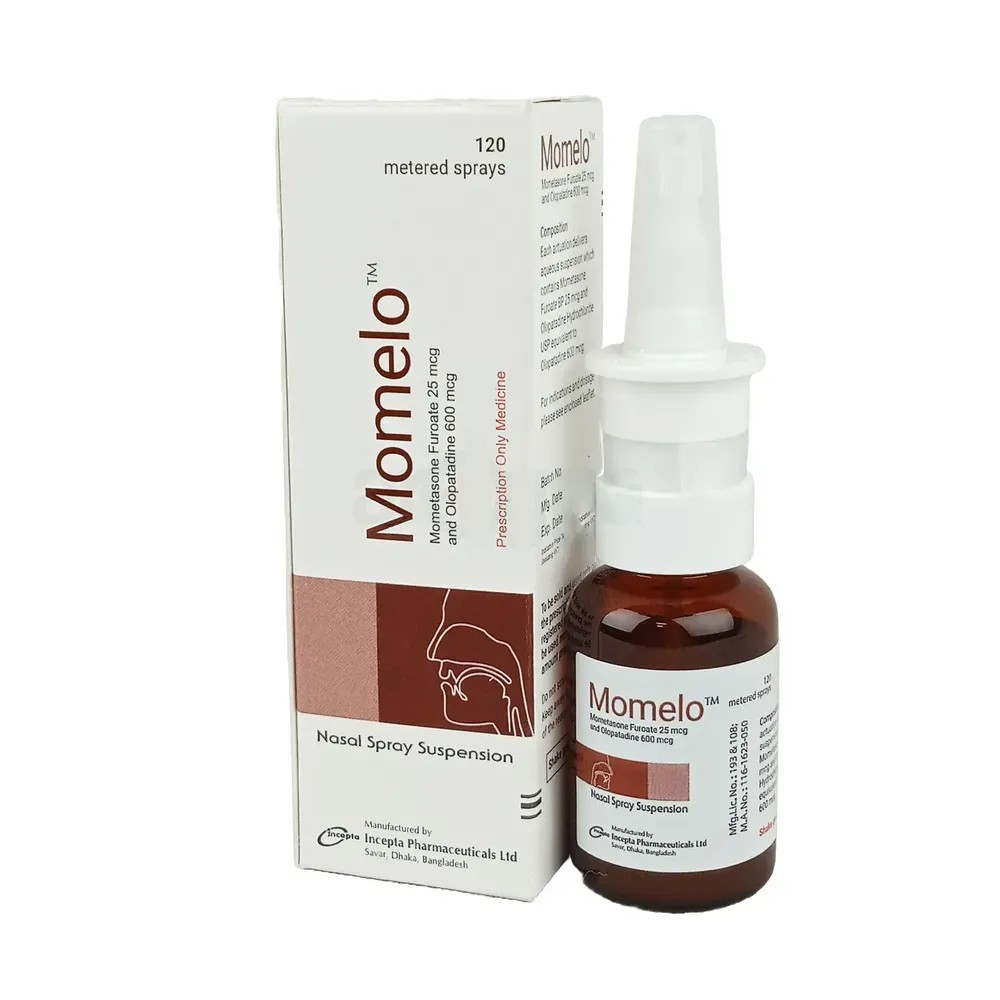
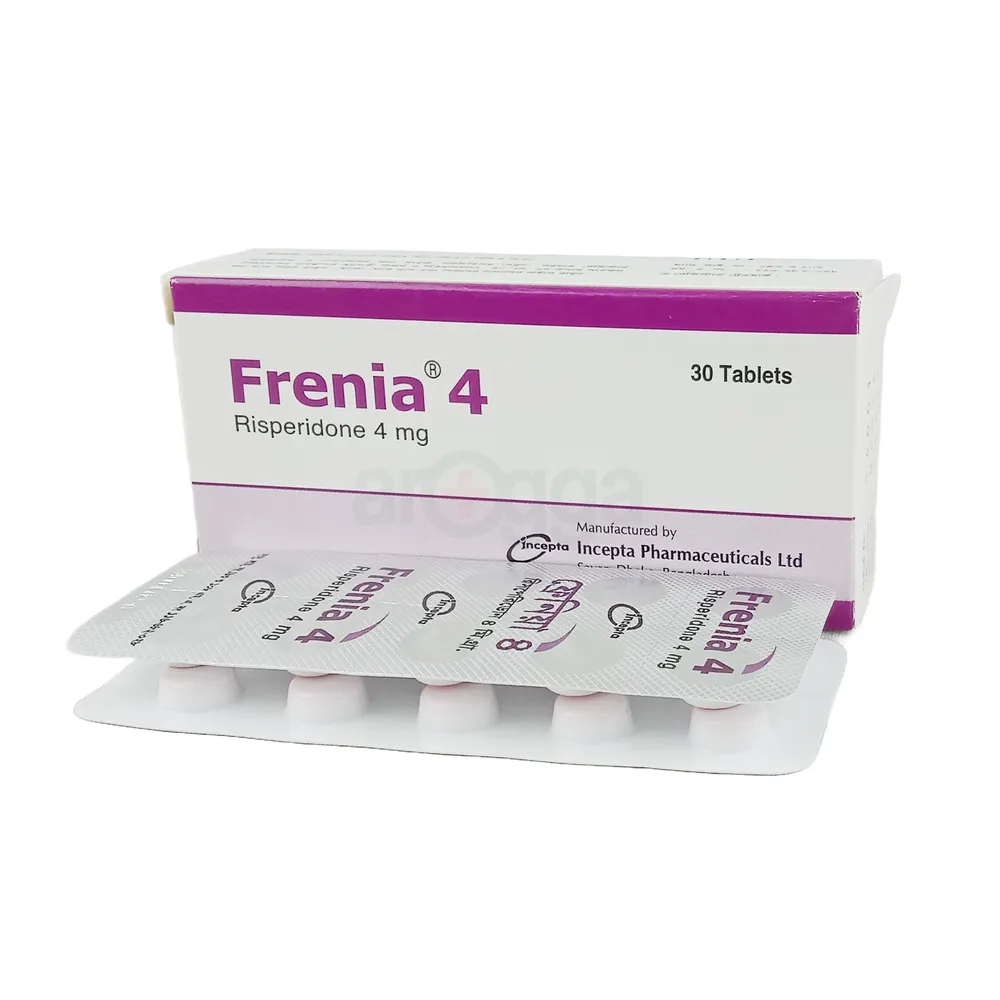
Description
Rynex 0.05% Nasal Drops, manufactured by Incepta Pharmaceuticals Ltd., contain Oxymetazoline Hydrochloride as the active ingredient. Oxymetazoline is a sympathomimetic amine that acts as a direct agonist at α-adrenoceptors, leading to rapid and long-acting vasoconstriction of the nasal arterioles. This reduces blood flow and diminishes mucosal swelling, thereby improving nasal airflow and sinus drainage. Rynex Nasal Drops are indicated for the symptomatic relief of nasal congestion associated with conditions such as acute or chronic rhinitis, the common cold, sinusitis, upper respiratory allergies, epistaxis (nosebleeds), and hay fever. It is also used as a nasal decongestant in otitis media. The recommended dosage for adults and children aged 6 years and older is 2–3 drops in each nostril twice daily for 3–5 days. Children under 6 years of age should not use this medication. Prolonged use beyond 3–5 days is not recommended to avoid rebound congestion. Rynex Nasal Drops should be used with caution in patients with hypertension, cardiovascular disease, hyperthyroidism, or those taking monoamine oxidase inhibitors (MAOIs). Common side effects may include local irritation, dryness of the nasal mucosa, or sneezing. As with all medications, it is essential to follow the prescribed dosage and consult a healthcare professional for personalized advice.





 400MG.jpg)




.webp)


.jpg)


.webp)















.jpg)




.jpg)





.jpeg)














.jpg)
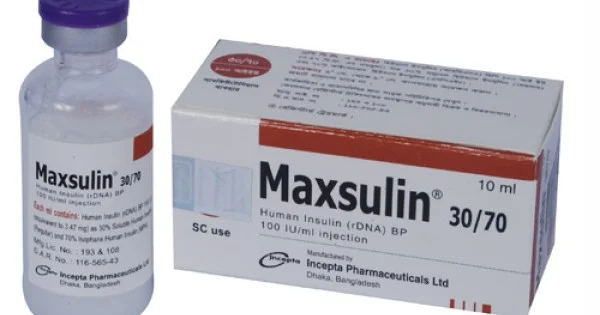
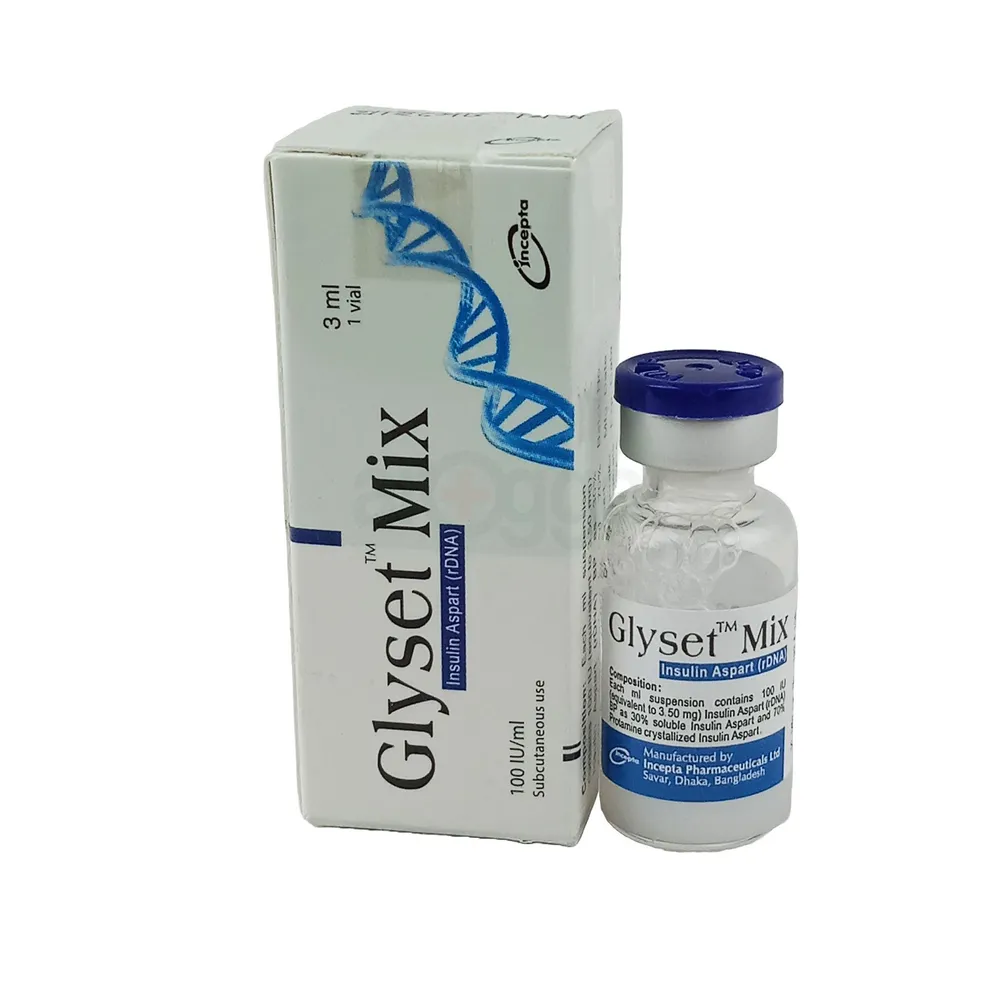
 100 IU 3 ML.webp)


.jpeg)
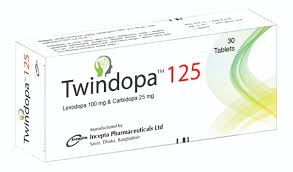
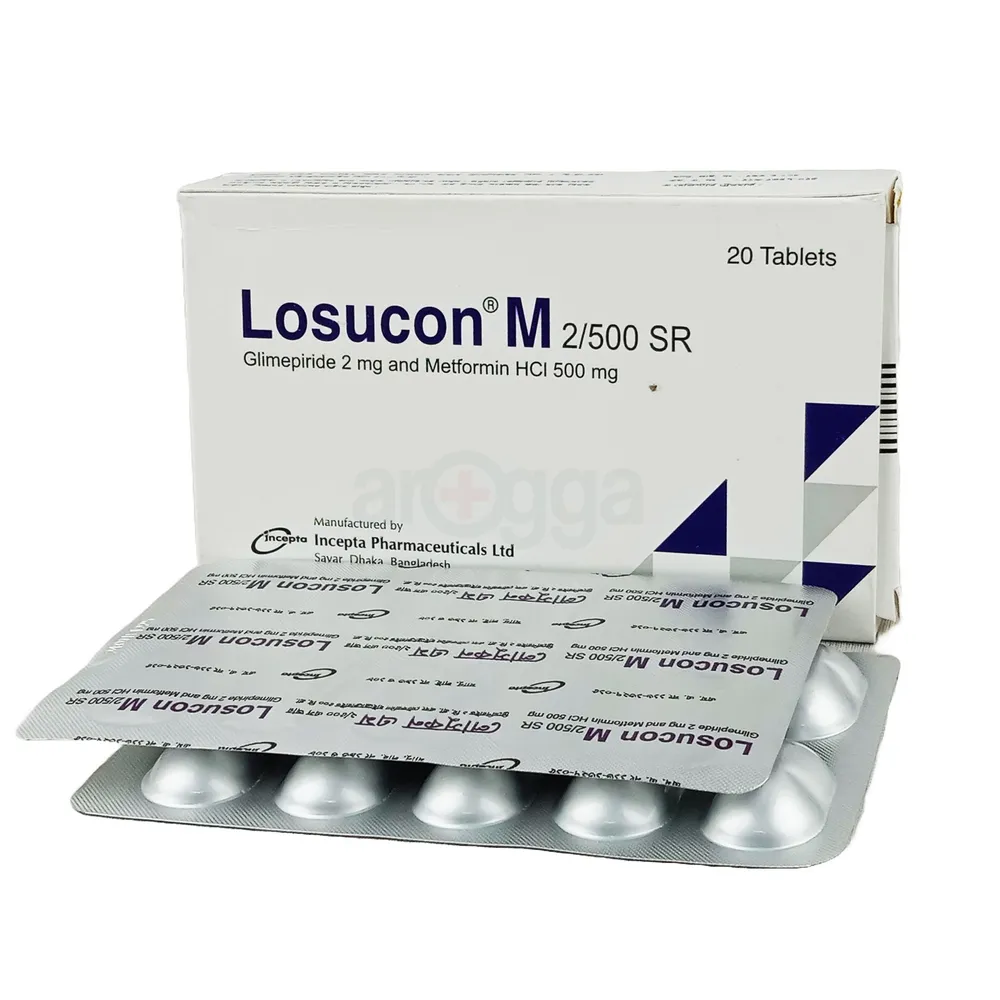
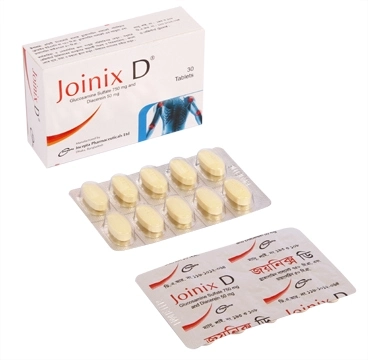

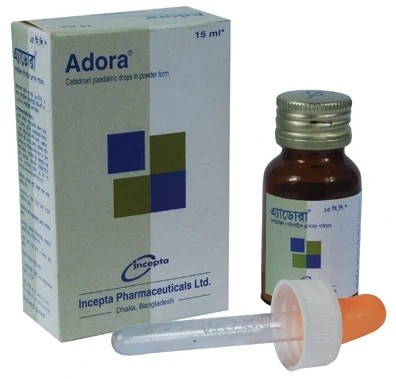





.jpg)

.jpeg)



















.webp)





















.jpg)
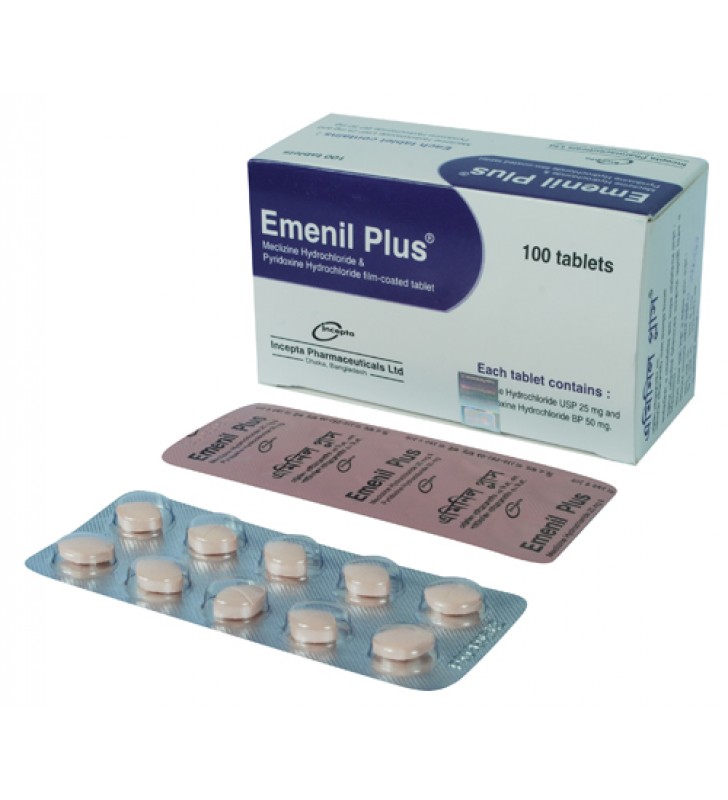
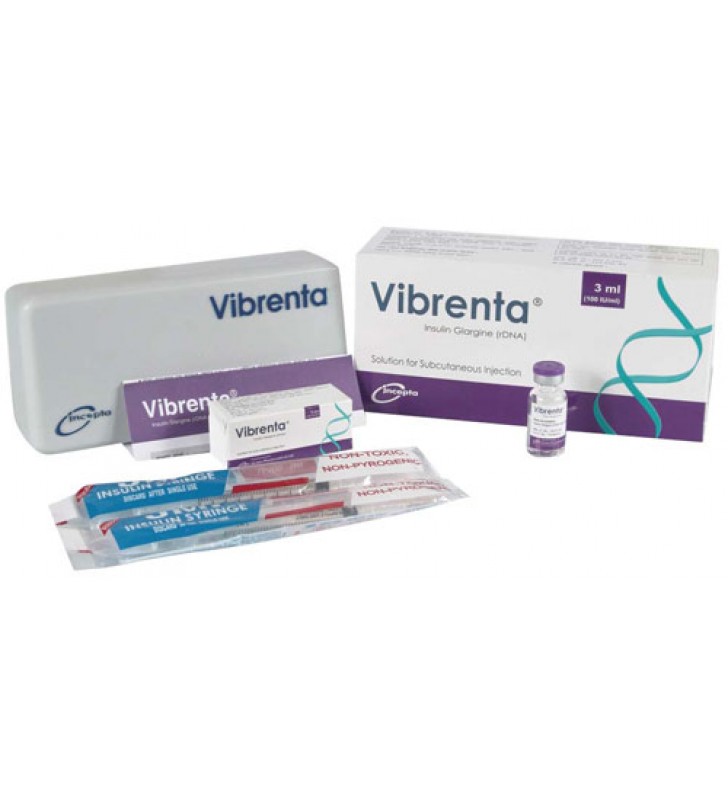
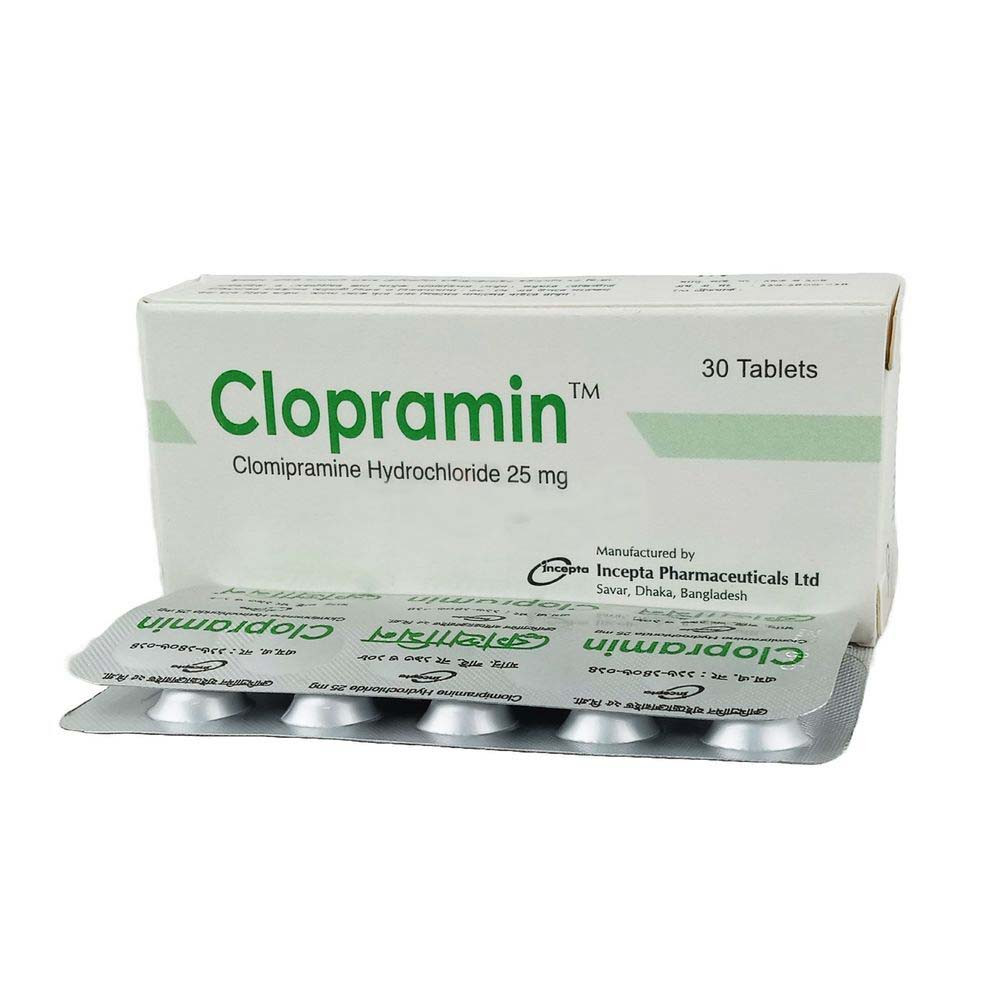





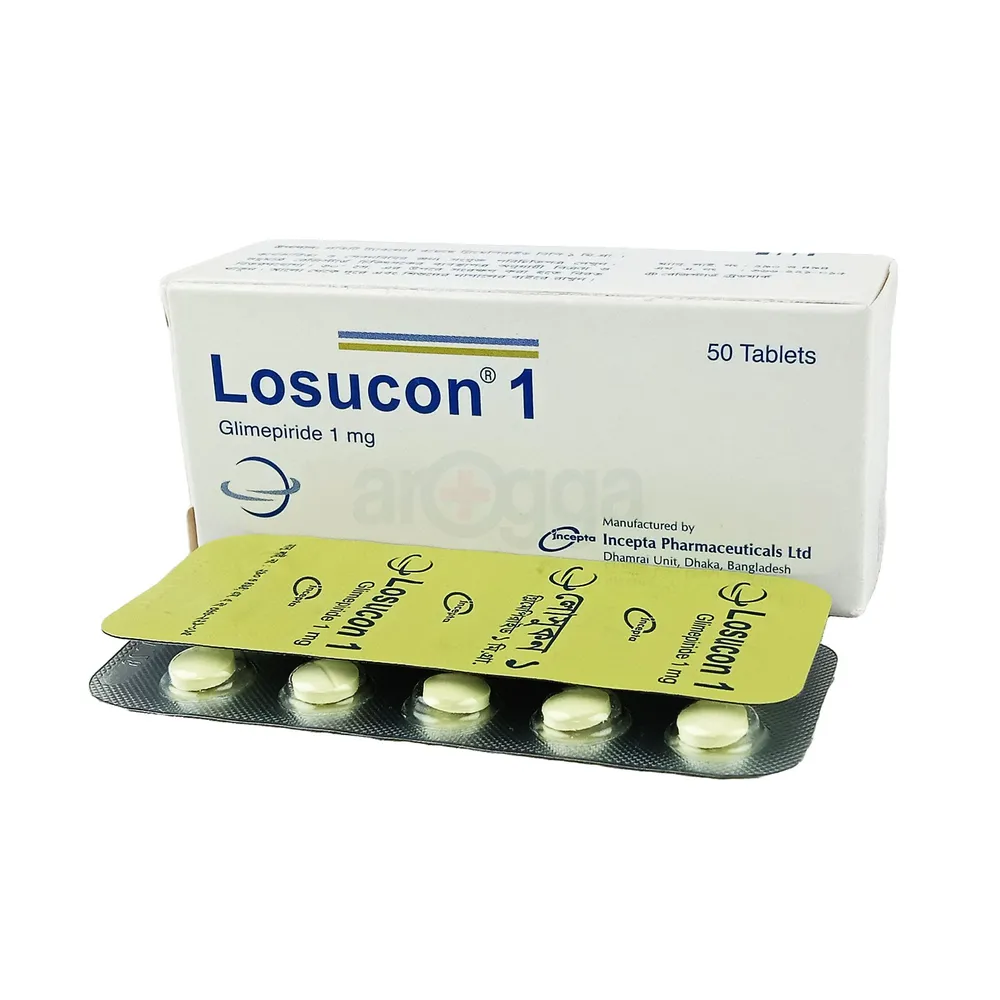









.jpg)






.jpg)















.jpeg)











.png)