Description
Sitagil M 50/1000 ER Tablet is an extended-release oral medication manufactured by Incepta Pharmaceuticals Ltd., combining Sitagliptin Phosphate Monohydrate (50 mg) and Metformin Hydrochloride (1000 mg). This combination is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both Sitagliptin and Metformin is appropriate. Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that enhances the body's own ability to lower blood sugar by increasing insulin release and decreasing glucagon levels in the circulation. Metformin, a biguanide, works by decreasing hepatic glucose production, decreasing intestinal absorption of glucose, and improving insulin sensitivity by increasing peripheral glucose uptake and utilization. Together, these medications provide better control of blood sugar levels. The recommended starting dose for patients not currently treated with Metformin is 50 mg Sitagliptin/500 mg Metformin hydrochloride twice daily, with gradual dose escalation recommended to reduce gastrointestinal side effects associated with Metformin. The maximum recommended daily dose is 100 mg Sitagliptin and 2000 mg Metformin. Sitagil M 50/1000 ER should be taken with food, preferably in the evening, to reduce gastrointestinal side effects. Patients should swallow the tablet whole and not crush, chew, or split it. Common side effects may include diarrhea, upper respiratory tract infection, and headache. It is important to monitor renal function periodically during treatment with Sitagil M 50/1000 ER. Patients should consult their healthcare provider before starting this medication to ensure it is appropriate for their condition











 400MG.jpg)










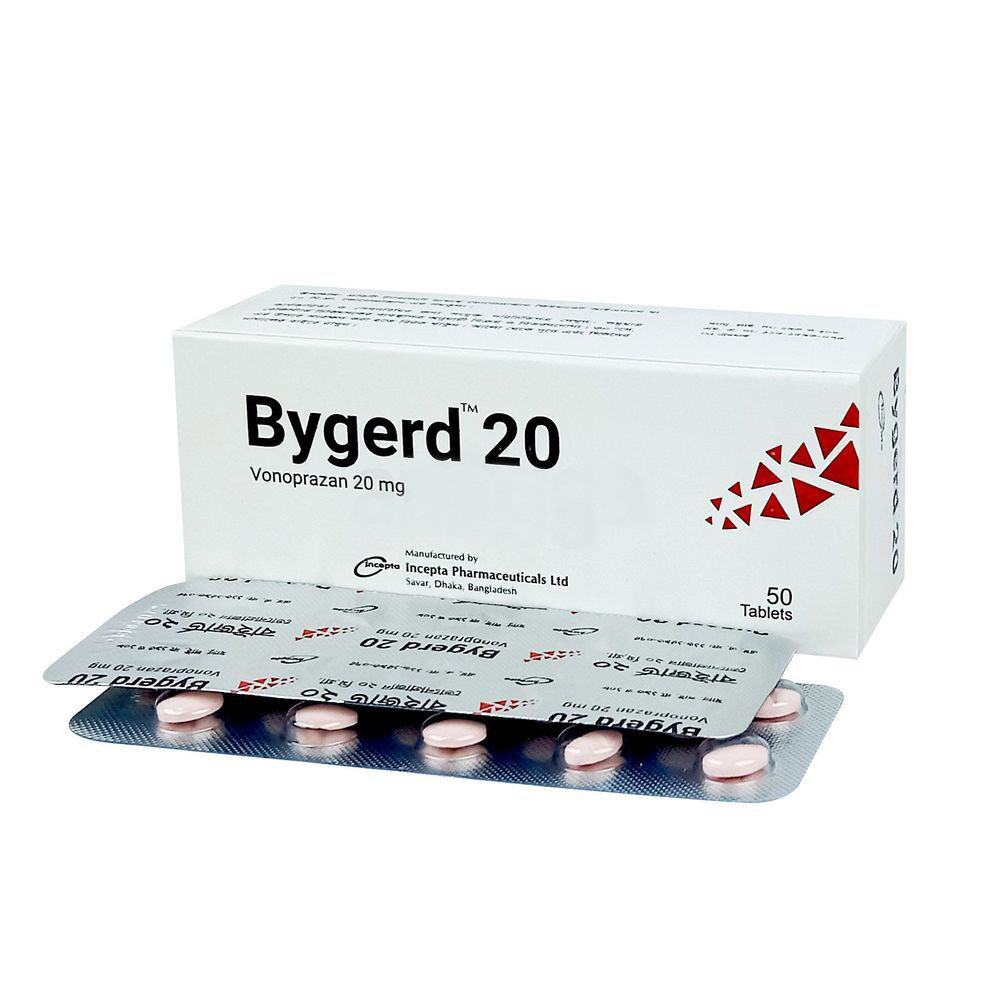
















.jpeg)









.jpg)
























.webp)













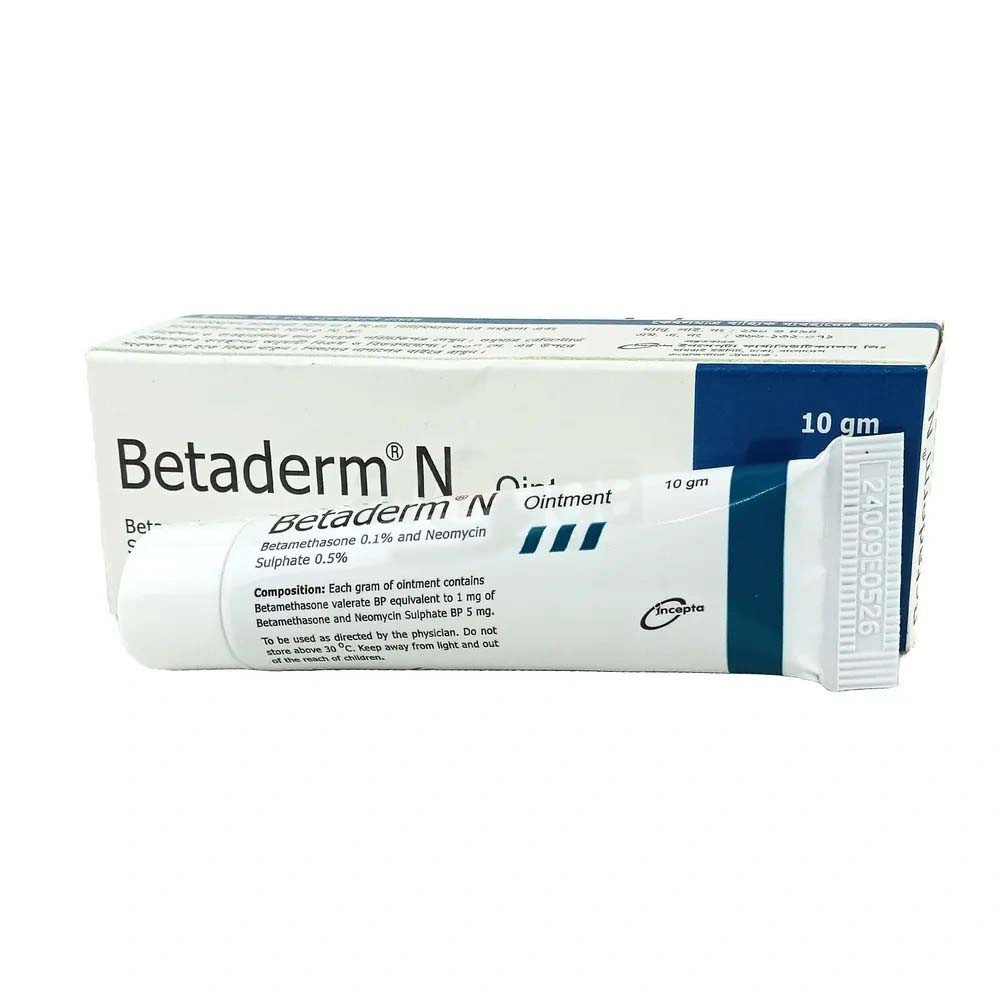
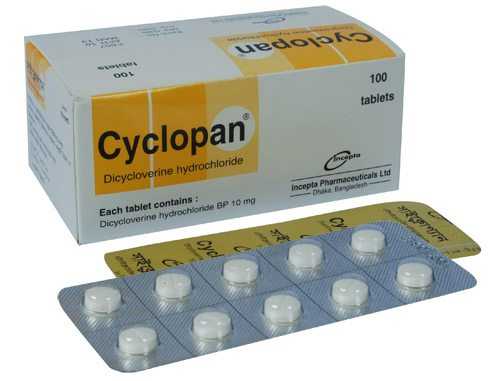
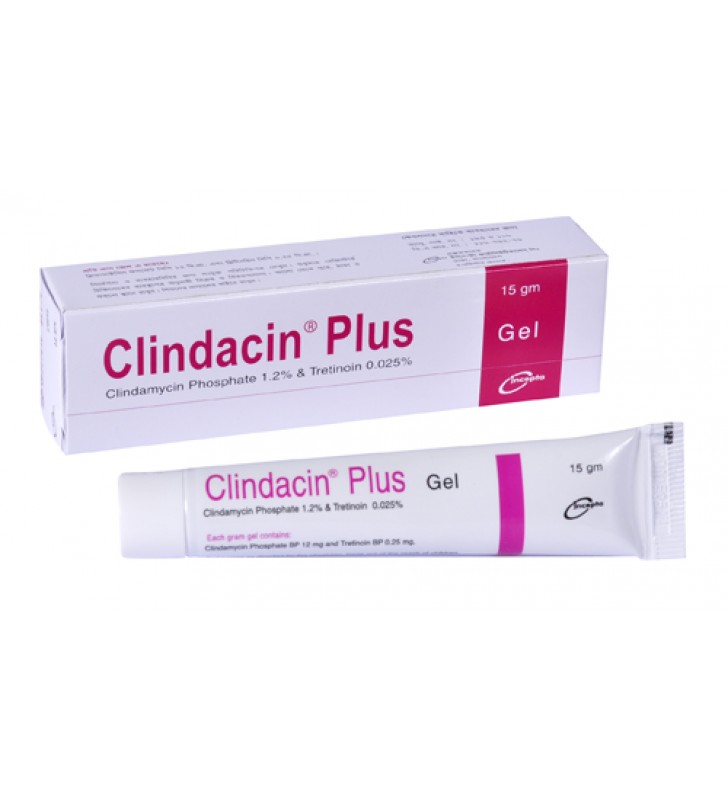
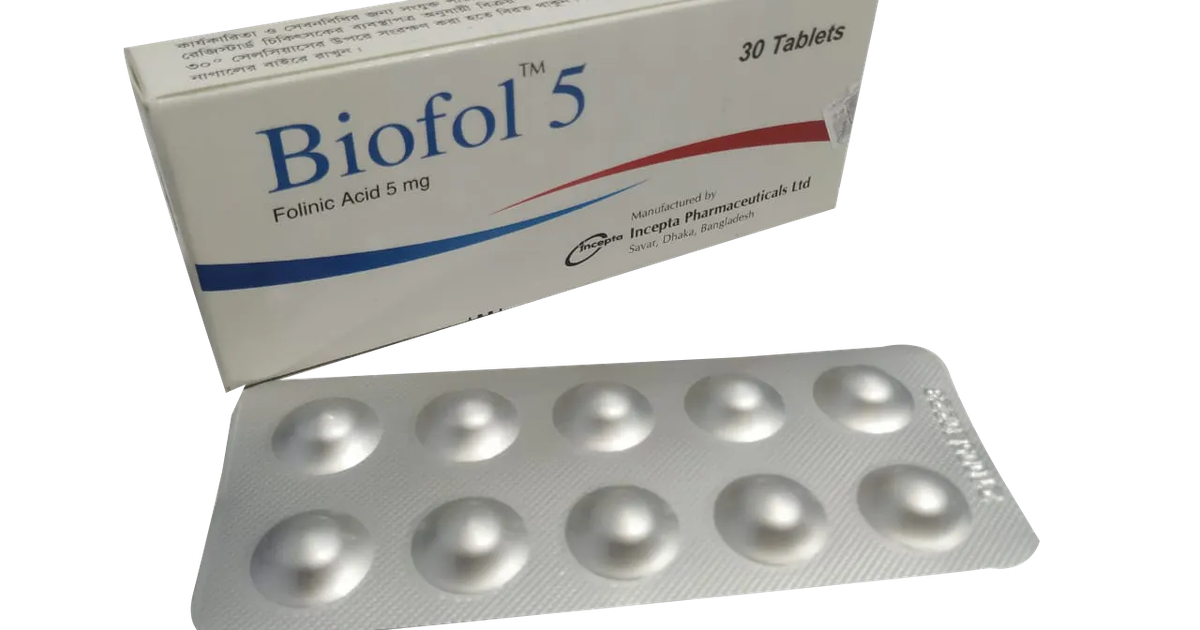









.webp)


.webp)




















.jpg)






.jpg)

.jpeg)
.png)

.jpeg)













.jpg)









.jpg)






.jpg)






.jpg)







.jpg)






.jpeg)






 100 IU 3 ML.webp)