Description
• রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন।
Indications:
Pronex-MUPS 20 mg Tablet is indicated for:
-
Gastroesophageal Reflux Disease (GERD)
-
Healing of erosive esophagitis
-
Maintenance of healing of erosive esophagitis
-
Symptomatic GERD
-
Reduction of NSAID-associated gastric ulcer
-
Helicobacter pylori eradication (triple therapy)
-
Zollinger-Ellison syndrome
-
Acid-related dyspepsia
-
Duodenal and gastric ulcers
Pharmacology:
Esomeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+/K+-ATPase enzyme system at the secretory surface of gastric parietal cells. This effect leads to inhibition of both basal and stimulated gastric acid secretion, irrespective of the stimulus. As the binding of esomeprazole to the H+/K+-ATPase enzyme is irreversible, new enzyme synthesis is required to resume acid secretion.
Dosage:
-
GERD (Without Erosive Esophagitis): 20 mg once daily for 4 weeks; consider an additional 4 weeks of treatment if symptoms do not resolve completely in the first 4 weeks.
-
GERD (With Erosive Esophagitis): 20–40 mg once daily for 4–8 weeks.
-
Helicobacter pylori Eradication (Triple Therapy): 20 mg twice daily in combination with amoxicillin and clarithromycin for 10 days.
-
Zollinger-Ellison Syndrome: 80 mg once daily; adjust dosage as needed.
-
NSAID-Associated Gastric Ulcer: 20–40 mg once daily for up to 6 months.
Administration:
Pronex-MUPS 20 mg Tablet should be taken on an empty stomach, at least one hour before meals. Swallow the tablet whole; do not chew, crush, or break it. For patients who have difficulty swallowing tablets, the tablet can be opened and the pellets mixed with a small amount of applesauce and swallowed immediately.
Interaction:
Esomeprazole may interact with drugs metabolized by the cytochrome P450 system, such as diazepam, warfarin, and phenytoin. It may also affect the absorption of drugs like ketoconazole, digoxin, and iron. Consult a healthcare provider before combining with other medications.
Contraindications:
-
Known hypersensitivity to esomeprazole or any component of the formulation.
-
Severe hepatic impairment (Child-Pugh C).
Side Effects:
Common side effects include:
-
Headache
-
Diarrhea
-
Nausea
-
Flatulence
-
Abdominal pain
-
Constipation
-
Dry mouth
Pregnancy & Lactation:
Esomeprazole is classified as FDA Pregnancy Category B. It is not known whether esomeprazole is excreted in human milk. Use during pregnancy and lactation should be considered only if the potential benefit justifies the potential risk to the fetus or infant.
Therapeutic Class:
Proton Pump Inhibitor
Storage Conditions:
Store at room temperature, away from moisture and heat. Keep out of reach of children. Do not use after the expiration date.


.jpeg)

 MR 30MG.jpg)
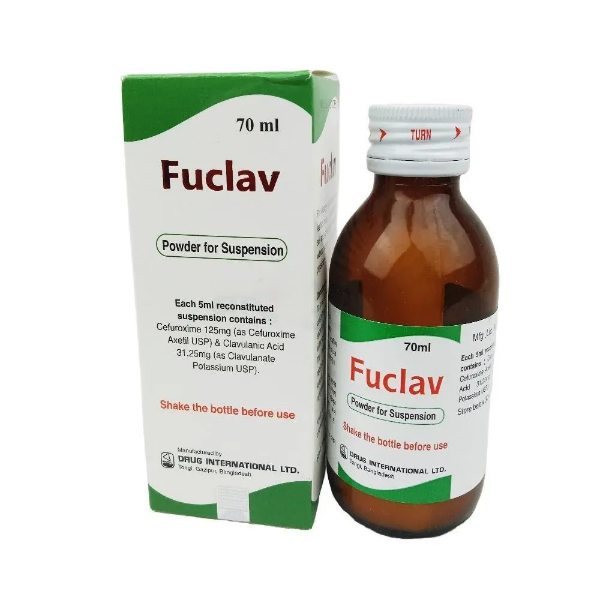
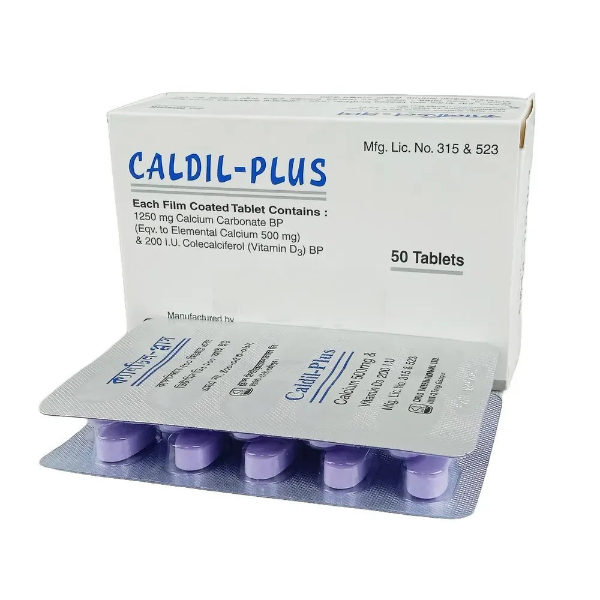
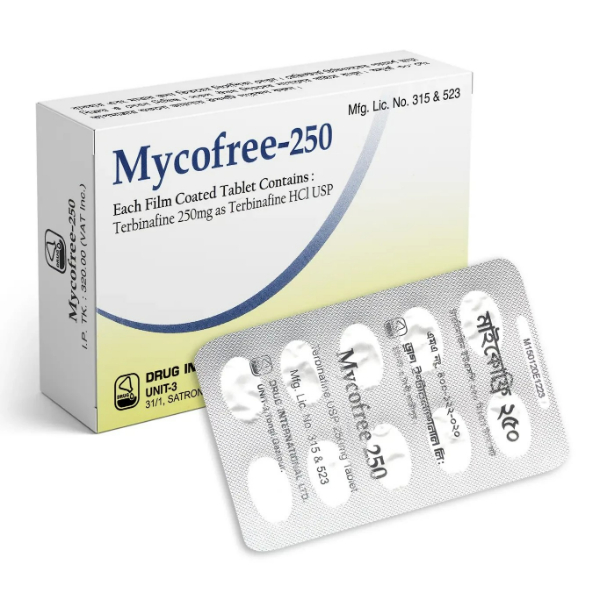
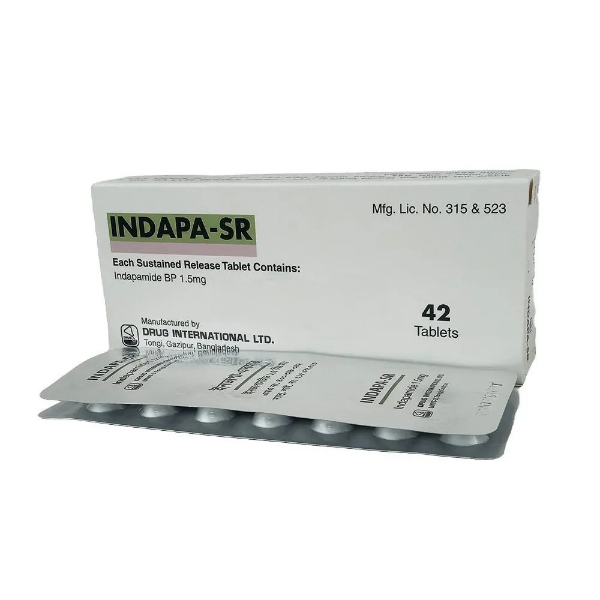


 PENSET 3ML.jpg)
.jpeg)

50 MG.jpg)




.jpeg)





850 MG.jpg)



 800 IU.jpg)


6MG.jpg)







 10ML.jpg)